Uncovering the crucial science behind bFGF
Source: PricellaPublished: 2024-11-11
As early as 1940, Hoftman and colleagues identified a substance in extracts from the brain and pituitary gland that promoted fibroblast growth. In 1974, this substance was successfully isolated and purified, earning the name Fibroblast Growth Factor (FGF). Subsequently, researchers isolated another substance highly homologous to FGF. Due to its rich content of acidic amino acid residues and an isoelectric point in the acidic range (pH = 5.6), it was designated as Acidic Fibroblast Growth Factor (aFGF). The originally discovered FGF, being sensitive to acids and heat, with an alkaline isoelectric point (pH = 9.6), was thus referred to as Basic Fibroblast Growth Factor (bFGF).
In this article, we will delve into an important member of the FGF family—bFGF—aiming to help you easily grasp the concepts and successfully conduct related experiments.
Decoding the Structural Blueprint of bFGF:
bFGF is a cationic polypeptide composed of 155 amino acids, known for its role in promoting cellular mitosis. Notably, 55% of its amino acid sequence is identical to that of aFGF, with a molecular weight ranging between 16 to 18.5 kDa. The structure of the bFGF molecule features four cysteine residues, which play a crucial role in forming its three-dimensional spatial conformation. Interestingly, the biological activity of recombinant bFGF remains unchanged even when serine replaces cysteine. Additionally, the single-chain polypeptide structure facilitates its expression in Escherichia coli.
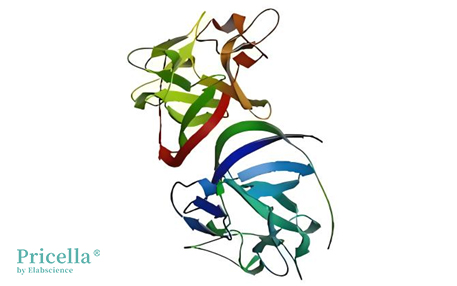
The Spatial Structure of bFGF (Image sourced from the Internet)
bFGF exhibits a strong affinity for heparin, particularly between amino acids 114 and 123, where it demonstrates high binding affinity; other regions show lower affinity. Monoclonal antibodies that block bFGF's interaction with its receptors do not affect its ability to bind heparin. However, the removal of the hydroxylated amino acid at position 42 in the bFGF molecule results in the loss of heparin affinity, potentially leading to a reduction in certain biological activities.

Interactions Between bFGF and Heparin[1]
Elucidating the Action Mechanisms of bFGF:
bFGF exerts its biological functions by binding to receptors on target cells. Consequently, the bFGF synthesized within the cell must be secreted into the extracellular environment to exert its effects. However, the mRNA translation products of bFGF lack the signal sequence necessary for directing their secretion, indicating that its secretion pathway deviates from the classical route. In addition to potentially being released following cellular damage or death, bFGF may also function through autocrine and paracrine signaling mechanisms.
Upon binding to its receptors, bFGF may transmit signals to the cell nucleus through the following pathways:
1. bFGF may activate adenylate cyclase and guanylate cyclase, leading to the phosphorylation of phospholipase C (PLC-β1). This activation results in the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DG) and inositol trisphosphate (IP3), subsequently activating protein kinase C (PKC) and triggering an influx of Ca²⁺.
2. After bFGF binds to its receptor, it may translocate to the nucleus, influencing the function of RNA polymerase I, thereby enhancing the transcription of nucleolar genes. This process facilitates the acceleration of cell transition from the G0 phase to the G1 phase, and from the G1 phase to the S phase, stimulating increased DNA synthesis and promoting cell division and proliferation.
3. bFGF may also form a complex with its receptor, FGFR, which subsequently transmits signals through an endocytic process.
Unleashing the Potential of bFGF in Cell Culture Applications
Leveraging bFGF for Cultivating Primary Endothelial Cells
In primary endothelial cell cultures, bFGF plays a crucial biological role. Typically used in conjunction with heparin, bFGF significantly enhances the proliferation of endothelial cells and promotes their migration. This synergy is vital for both the proliferation and morphological maintenance of endothelial cells.
Harnessing bFGF for Enhanced Stem Cell Cultivation
bFGF is a key growth factor in stem cell cultures, serving not only as a critical mitogenic factor but also as an inducer of morphogenesis and differentiation.
bFGF is essential for cell proliferation and the maintenance of an undifferentiated state. During the culture of murine stem cells, the addition of Leukemia Inhibitory Factor (LIF) typically effectively suppresses stem cell differentiation. However, in human stem cell cultures, the use of bFGF is necessary to inhibit cell differentiation. When employing feeder layer cells for culture, the bFGF secreted by these feeder cells also plays a similar role.
Unveiling the Role of bFGF in Neuronal Cell Functionality
bFGF is a crucial mitogenic factor that stimulates the proliferation of neural stem cells. For instance, in experiments involving subcutaneous injections of bFGF in young mice, a significant increase in the proliferation of neural stem cells was observed in neurogenic regions, such as the hippocampus and the granule layer of the cerebellum. Additionally, bFGF works synergistically with factors such as Insulin-like Growth Factor (IGF) and Platelet-Derived Growth Factor (PDGF) to co-regulate neural stem cell proliferation.
However, bFGF not only promotes the proliferation of neural stem cells but also influences their differentiation. During the process of proliferation, bFGF suppresses the differentiation of neural stem cells. When the concentration of bFGF is reduced, neural stem cells can differentiate into neurons, glial cells, and oligodendrocytes, indicating that bFGF plays a pivotal regulatory role in the differentiation process of neural stem cells.
Furthermore, bFGF may promote neural regeneration and repair in neural cell cultures. Given that bFGF is known to activate the renewal of all layers of the skin and promote fibroblast growth, it is hypothesized that bFGF may have similar effects in neural cell cultures, aiding in the regeneration and repair of neural cells.
References
[1] Song X, Fan Z, Tian Z, et al. Phase separation on cell surface facilitates bFGF signal transduction with heparan sulphate[J]. Nature Communications, 2022, 13(1):1112-1112.
Prev: Exploring Microvascular Endothelial Cells: Core Concepts and Understanding





