Exploring Microvascular Endothelial Cells: Core Concepts and Understanding
Source: PricellaPublished: 2024-11-11
Microvascular endothelial cells (MVECs) form the physical barrier between blood and tissue fluids and play crucial roles in coagulation, anticoagulation, inflammation, anti-inflammation, and immune response. Their in vitro culture models are widely used in related research. Due to the significant heterogeneity between MVECs from different tissues and organs, and because MVECs exhibit morphological, phenotypic, and functional differences based on their anatomical locations, endothelial cells from different sources are not interchangeable. Major types include endothelial cells from the intestine, lungs, heart, brain, choroid, retina, and dermal microvasculature.
In this session, we provide a comprehensive overview of methods and steps for the isolation, culture, and identification of MVECs to help you quickly set up your experiments.
Isolation Methods
There are three primary methods for isolating and culturing MVECs: enzymatic digestion, tissue explant culture, and immunomagnetic bead separation.
1. Enzymatic Digestion Method
This method uses digestive enzymes to dissociate cellular matrices such as collagen, fibrin, and other extracellular components from the tissue, breaking the cells into a single-cell suspension for further culture.
Procedure: Select the experimental animal and age (depending on the tissue source) → Tissue sampling from the specific site → Tissue mincing and washing → Enzymatic digestion → Centrifugation to remove enzymes → Cell counting and seeding → Cell purification → Cell identification.
2. Tissue Explant Culture Method
In this approach, tissues are cut into small pieces and placed in culture flasks for growth.
Procedure: Select the experimental animal and age (based on the tissue source) → Tissue sampling → Tissue mincing → Seeding for culture.
3. Immunomagnetic Bead Separation
This technique involves coating magnetic beads with antibodies to select or deplete labeled cells through antigen-antibody reactions, enabling positive or negative selection.
Procedure: Select the experimental animal and age (based on the tissue source) → Tissue sampling → Tissue mincing and washing → Enzymatic digestion → Centrifugation to remove enzymes → Incubate cells with magnetic beads → Magnetic bead separation → Cell collection and counting → Seeding for culture.
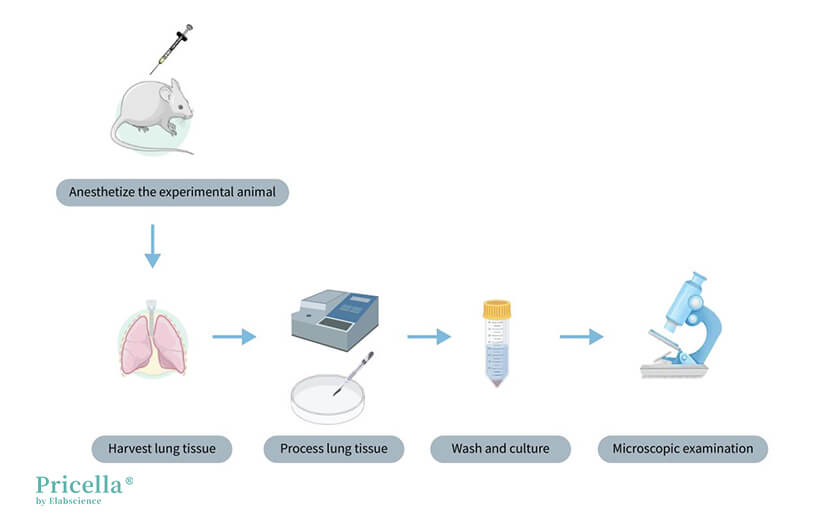
Illustration of Rat Lung Microvascular Endothelial Cell Isolation
Advantages and Disadvantages of Each Isolation Method
1. Enzymatic Digestion Method: Quickly yields a large number of cells but is labor-intensive, prone to contamination, requires multiple purification steps, and involves expensive enzymes like collagenase, increasing costs. Optimal enzyme concentration and digestion time need adjustment.
2. Tissue Explant Culture Method: Has a high success rate and low cost, suitable for primary cultures with limited tissue. However, cell migration timing is unpredictable, and incomplete removal of tissue pieces may lead to contamination with other cell types.
3. Immunomagnetic Bead Separation: Produces high-purity cells but is costly and requires a larger cell quantity for selection and purification.
Important Considerations
1. The age and health of experimental animals can affect cell viability and passage numbers.
2. Tissue dissection should minimize extraneous tissue and ensure sterility.
3. Obtaining appropriately sized tissue pieces that easily adhere to surfaces is crucial for cell isolation.
4. Parameters such as pipette size and the number of pipetting actions directly impact cell adherence and purity.
5. Although enzyme activity varies by tissue, digestion conditions must be optimized based on the specific cell type being isolated.
6. Controlling digestion time and temperature is essential; excessive time or high temperatures can hinder cell adhesion, while insufficient digestion fails to separate tissues adequately.
7. Use endothelial cell-specific culture media containing essential trace elements and growth factors.
8. Surface treatments (e.g., PLL, gelatin) are recommended to promote endothelial cell adhesion and 3D morphology.
9. Compared to mouse cells, rat cells of the same type tend to have slightly higher passage numbers.
10. With increasing passages, cell morphology may change.
Identification Markers
1. Microscopic Examination
Initial assessment of cell morphology and growth patterns reveals characteristic cobblestone, polygonal, or spindle-like shapes with large, clear nuclei.
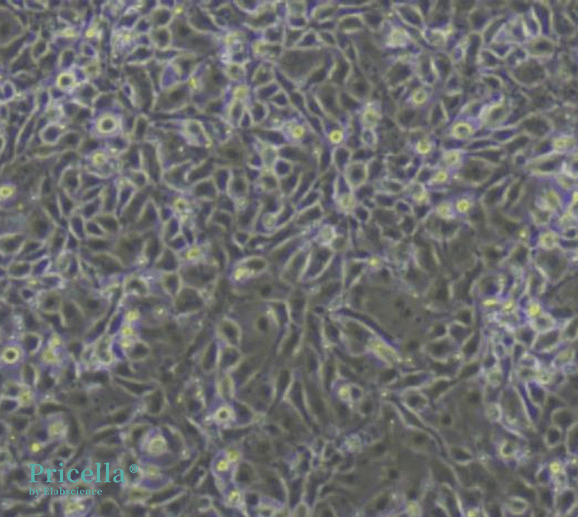
Microvascular endothelial cells obtained through the tissue explant method exhibit a cobblestone-like appearance

After obtaining microvascular endothelial cells using the explant method, digestion and subculturing can lead to changes in cell morphology
Specific Markers
Commonly used markers include positive expression of Factor VIII, vWF, and CD31.
Prev: Optimized Protocols for K7M2-WT Cell Culture and Preservation





