Technical Principles and Procedures for Cell Cryopreservation and Resuscitation
Source: PricellaPublished: 2024-10-23
Cell cryopreservation and resuscitation are routine procedures in cell culture processes.
Cell cryopreservation refers to the technique of storing cells in an ultra-low temperature environment, allowing them to temporarily "hibernate" and be revived when needed.
Cell resuscitation involves rapidly thawing cells stored at -80°C or in liquid nitrogen and allowing them to resume growth.
This article will introduce the key techniques and procedures for cell cryopreservation and resuscitation.
Cell Cryopreservation Section
1. Cryopreservation Principles
The principle of cell cryopreservation is "slow freezing," which means gradually freezing the cells under slow cooling conditions.
If cell suspensions are rapidly frozen at ultra-low temperatures, cells may suffer freeze damage and die. The protectants added in the cryopreservation solution (such as DMSO and glycerol) and the isopropanol in the programmed freezing containers help achieve controlled cooling.
2. Precautions for Using DMSO
DMSO can quickly penetrate cell membranes, enter cells, slow down the rate of temperature decrease, and reduce the formation of ice crystals within the cells, thus protecting the cells.
It is important to note that when DMSO dissolves in the culture medium, it releases a significant amount of heat. Therefore, the freezing medium should be prepared in advance, and DMSO should not be directly added to the cell-containing culture medium to avoid thermal injury to the cells.
Additionally, since DMSO is a carcinogen, it is essential to wear gloves and follow proper handling procedures.
3. Formulation of Freezing Medium
The components of freezing medium generally include a basal culture medium, fetal bovine serum, and DMSO. The serum proportion ranges from 10% to 90%, and the DMSO proportion ranges from 5% to 10%. Based on the cell culture experience from Pricella’s Laboratory, the recommended freezing medium ratio is 55% basal culture medium + 40% fetal bovine serum + 5% DMSO. For difficult-to-culture cells, a ratio of 90% fetal bovine serum + 10% DMSO can be used.
4. Cell Cryopreservation Density
During cell cryopreservation and resuscitation, some cell loss is inevitable, so the cryopreservation density should not be too low. Increasing the cryopreservation density helps improve the survival rate of resuscitated cells. The recommended density is 2-5 million cells/mL.
5. Cryopreservation Procedure
Method 1: Using a Programmed Freezing Container
Using a programmed freezing container is the most common cryopreservation method. Some commercially available freezing containers require the addition of isopropanol, while others do not.
The programmed freezing container should be brought to room temperature before use.
According to the cell culture experience at Pricella's Laboratory, using a programmed freezing container provides excellent cryopreservation results. If you don't have access to a programmed freezing container, please refer to Methods 2 and 3 below.
Operation Steps
Step 1: Prepare the freezing medium and set it aside at room temperature or pre-cool it at 4°C.
Step 2: Centrifuge to collect cells in the logarithmic growth phase (for adherent cells, digest and centrifuge; for suspension cells, directly centrifuge at 1200 rpm or 250×g for 3 minutes).
Step 3: Resuspend the cells in the prepared freezing medium and aliquot them into cryovials at 0.5-1.0 mL per vial. Tighten the vial caps and label them properly.
Step 4: Place the cryovials into the programmed freezing container and store the programmed freezing container in a -80°C freezer overnight (24 hours).
Step 5: Transfer the cryovials from the programmed freezing container to liquid nitrogen for long-term storage.
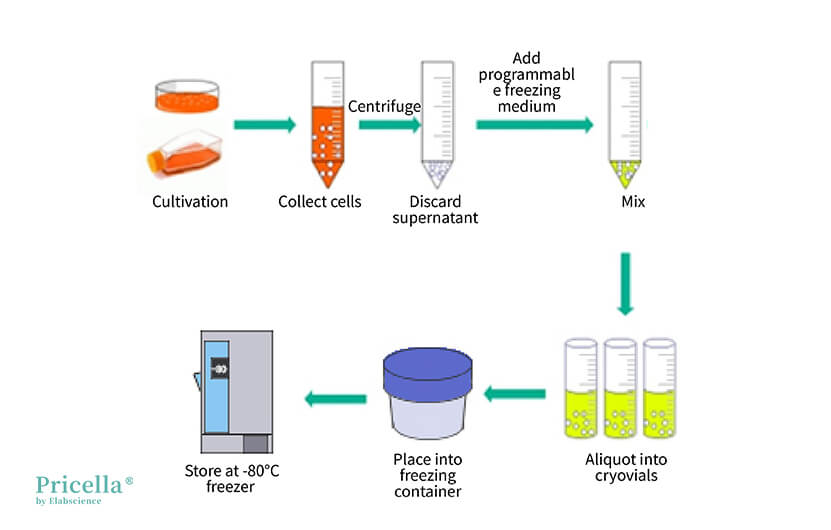
▲ Illustration of Operating with a Programmed Freezing Box
Method 2: Using Serum-Free Non-Programmable Freezing Medium
Serum-free non-programmable freezing medium is a commercial cryopreservation medium that requires no preparation and no freezing box, greatly enhancing convenience.
However, not all cells are suitable for this type of cryopreservation medium. A cryopreservation test must be conducted before bulk application.
Operation Steps
Step 1: Take the serum-free non-programmable freezing medium out of the refrigerator and set it aside.
Step 2: Centrifuge to collect cells in the logarithmic growth phase (for adherent cells, digest and centrifuge; for suspension cells, directly centrifuge at 1200 rpm or 250×g for 3 minutes).
Step 3: Discard the supernatant and add an appropriate amount of serum-free non-programmable freezing medium to resuspend the cells.
Step 4: Aliquot the cell suspension into cryovials at 0.5-1.0 mL per vial. Tighten the vial caps and label them properly.
Step 5: Transfer the cryovials directly into a -80°C freezer and move them to liquid nitrogen for long-term storage after 24 hours.
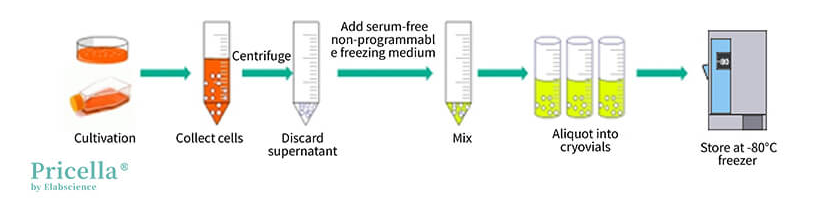
▲ Illustration of Operating with Serum-Free Non-Programmable Freezing Medium
Method 3: Manual Gradient Cooling
In the absence of a programmed freezing container or serum-free non-programmable freezing medium, manual gradient cooling can be chosen. However, manual gradient cooling is not suitable for all cells and yields inconsistent results, so a cryopreservation test is required first.
Operation Steps
Step 1: Prepare the freezing medium and set it aside at room temperature or pre-cool it at 4°C.
Step 2: Centrifuge to collect cells in the logarithmic growth phase (for adherent cells, digest and centrifuge; for suspension cells, directly centrifuge at 1200 rpm or 250×g for 3 minutes).
Step 3: Resuspend the cells in the prepared freezing medium and aliquot them into cryovials at 0.5-1.0 mL per vial. Tighten the vial caps and label them properly.
Step 4: Place the cryovials sequentially at 4°C for 20 minutes, then at -20°C for 30 minutes, then at -80°C for 24 hours, and finally transfer them to liquid nitrogen for long-term storage.
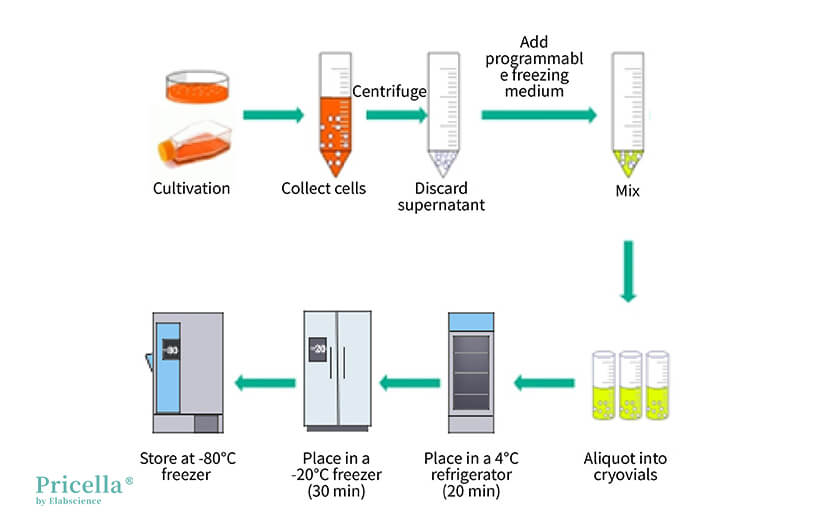
▲ Illustration of Manual Gradient Cooling Operation
Cell Resuscitation Section
Cell resuscitation emphasizes "rapid thawing"; the faster the cells thaw, the less damage they will incur. The process of cell resuscitation is not complicated, but each step must be performed meticulously to maximize the survival rate.
Cell Resuscitation Steps:
Step 1: Preheat the water bath to 37°C.
Step 2: Prepare a 15 mL centrifuge tube and add an appropriate amount of culture medium.
Tip: Add 4.5-9 mL of culture medium to the centrifuge tube. For more difficult-to-culture cells, the amount of culture medium can be increased accordingly.
Step 3: Remove the cryovial from the -80°C freezer or liquid nitrogen, place it in a polyethylene (PE) glove, and quickly immerse it in the water bath.
Tip: If the distance between the freezer or liquid nitrogen and the water bath is more than 1 minute by foot, place the cells on dry ice before transporting them to the water bath. Holding the cryovial in your hand or pocket might cause a gradual temperature rise, leading to ice crystal damage. Placing the cryovial in a PE glove helps prevent contamination.
Step 4: Vigorously shake the cryovial to ensure it thaws within 1 minute.
Tip: Thawing quickly is crucial, so using a timer is recommended. If thawing is not complete within 1 minute, it could be due to an excessive volume of freezing medium or insufficient shaking.
Step 5: Wipe off the water, transfer the cryovial to a biological safety cabinet, and use a pipette to slowly add the cell suspension to the centrifuge tube prepared in step 2.
Tip: When resuscitating multiple cryovials simultaneously, ensure not to mix them up.
Step 6: Centrifuge at 1200 rpm for 3 minutes.
Step 7: Discard the supernatant, resuspend the cells in fresh culture medium, and seed them into a new sterile culture vessel.
Tip: Centrifugation removes DMSO. This step is crucial for cells sensitive to DMSO. If the cells are not sensitive to DMSO, steps 6 and 7 can be skipped. Instead, directly adjust the culture medium volume to 10 mL and seed the cells into the culture vessel, replacing the medium the next day.
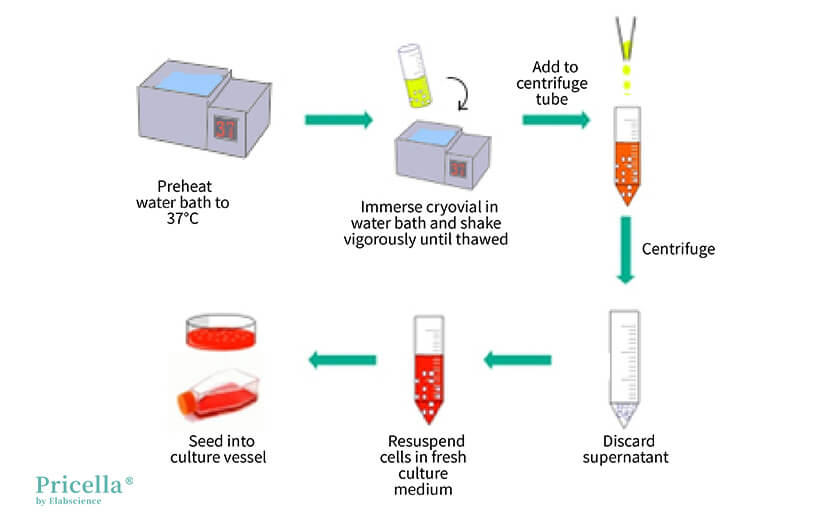
▲ Illustration of Cell Resuscitation Operation
After completing step 7, the cells will be resuscitated. These cells require some time to recover. If the cell density is below 80%, avoid changing the medium for the first 48 hours. Wait until the cell morphology and growth rate normalize before proceeding with passaging or other procedures. (Do not discard the cells if their condition appears poor within the initial two to three days post-resuscitation; patience is key, and it may take up to a week for recovery.)
However, if the cells recover quickly, you can commence subsequent cell culture experiments earlier.
Prev: How to Address Morphological Changes and Dissociation Issues in bEnd.3 Cells





