How to Address Morphological Changes and Dissociation Issues in bEnd.3 Cells
Source: PricellaPublished: 2024-09-09
bEnd.3 [BEND3] (mouse brain microvascular endothelial cells) are endothelial cells isolated from the brain tissue of mice with endothelial tumors. These cells are transformed by infection with an NTKmT retroviral vector expressing the polyoma virus middle T antigen. During culturing, the most common issues encountered with bEnd.3 cells are morphological changes and difficulty in dissociation. In this session of Cell Culture Academy, we will help you address these problems one by one.
Morphological Issues
bEnd.3 cells inherently grow slowly. At low density, they exhibit irregular cell morphology, while at high density, they present a regular fibrous appearance. If abnormal cell morphology is observed during culturing, it may be due to low cell density. It is recommended to passage the cells when they reach higher confluency. Below are images of bEnd.3 cells cultured at different densities:
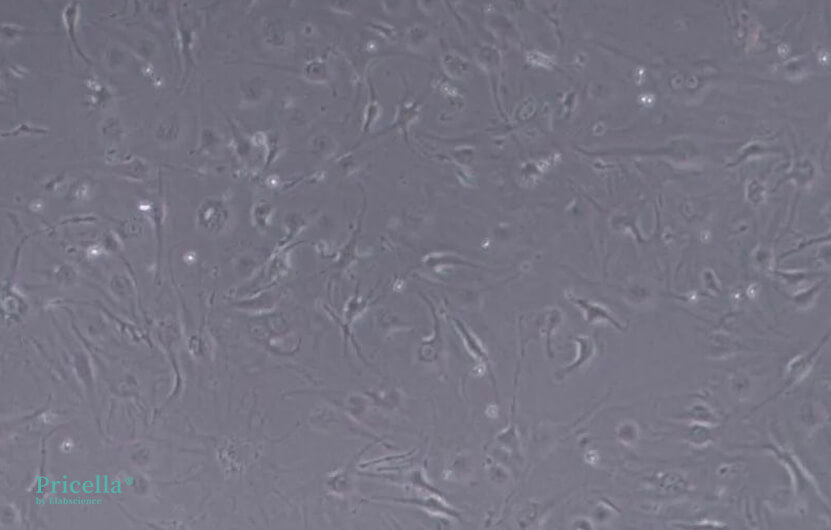
Low density (100x magnification)
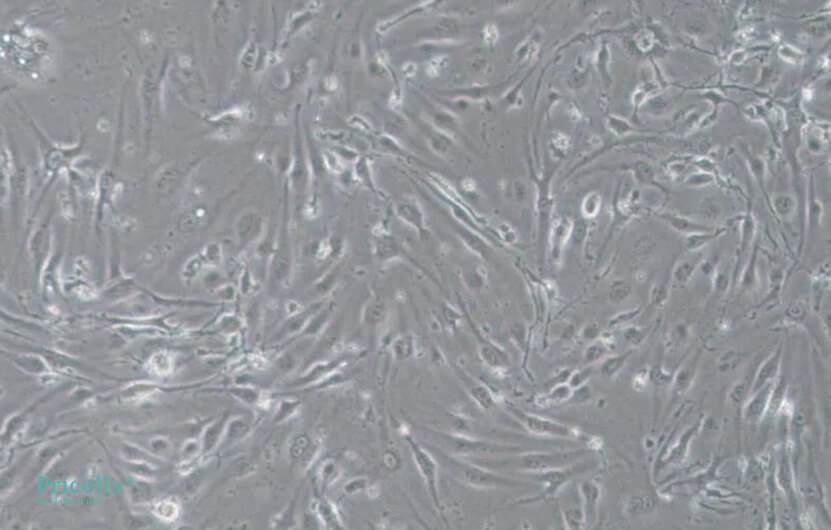
Medium density (100x magnification)
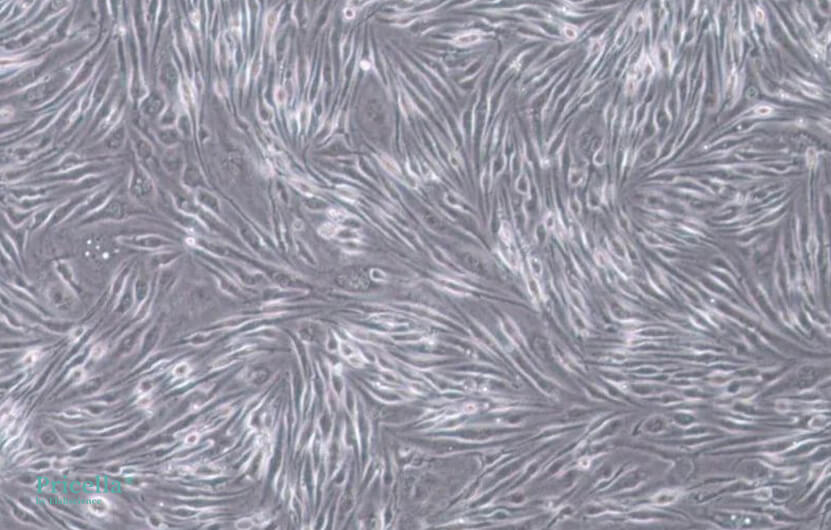
High density (100x magnification)
Dissociation Issues
Dissociation Time
The longer bEnd.3 cells are cultured, the more difficult they become to dissociate. For cells cultured for 4 days, dissociation typically takes 3-5 minutes; for those cultured for 7 days, dissociation usually takes 5-10 minutes. The exact dissociation time should be determined by observing the cells under a microscope.
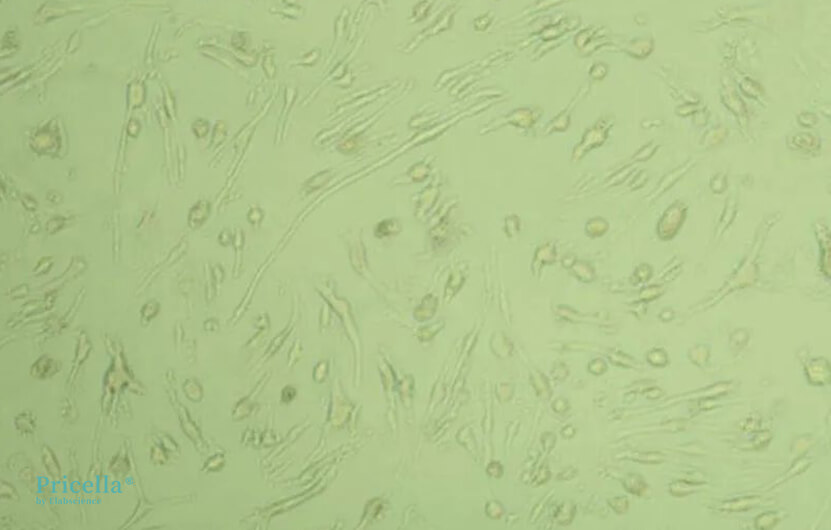
Cultured for 7 days, dissociated for 5 minutes microscopic image
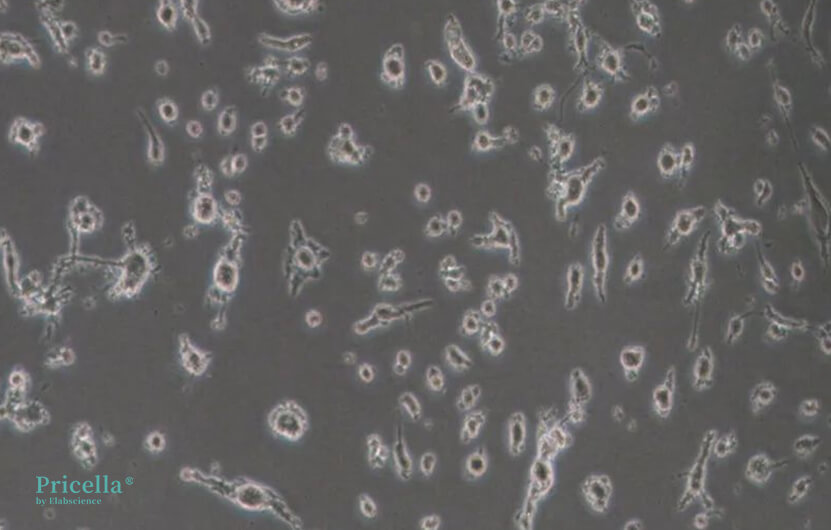
Cultured for 7 days, dissociated for 10 minutes microscopic image
Handling Difficult dissociation
If dissociation is difficult, there's no need to worry. You can solve this by performing dissociation in steps. Here’s how to do it (using a T25 flask as an example):
1. Remove the original culture medium.
2. Add approximately 2 mL of PBS and gently shake the flask to rinse the cells, then remove and discard the PBS.
3. Add about 1 mL of trypsin and gently shake the flask to ensure all cells are covered.
4. Place the flask in the incubator for dissociation, typically 3-5 minutes (adjust as necessary based on cell condition). When cells start to float under the microscope, add 2 mL of PBS, shake the flask to suspend the cells, but do not pipette the remaining adherent cells.
5. Transfer the PBS and cell suspension to a sterile centrifuge tube, add 3 mL of serum-containing medium to stop the dissociation, and gently pipette to create a single-cell suspension.
6. Add 0.5 mL of trypsin to the original culture flask, shake to cover the cells, and continue dissociation in the incubator until the cell clumps shrink and round up, detaching with gentle shaking.
7. Stop the dissociation by adding 3 mL of serum-containing medium, pipette all cells from the flask bottom, and gently pipette to create a single-cell suspension.
8. Combine the cell suspension with the previously collected cells in one centrifuge tube.
9. Centrifuge to collect the cell pellet at 1200 rpm for 3 minutes, discard the supernatant.
10. Add fresh culture medium, mix the cells by pipetting, and seed them into new culture flasks as needed, add the appropriate amount of culture medium, and incubate with a loosened cap or a vent cap.
11. Check the incubator’s CO2 level, temperature, and water tray.
Culturing Tips
1. Cell morphology varies during culturing; at low density, cells may appear radial and seem to cover the flask bottom but are actually sparse. Passage the cells when they are densely packed.
2. Avoid frequent medium changes; change the medium every 2-3 days or perform a half-medium change.
3. About 10% of live single cells may float during culturing. When adherent cell density is low, collect the floating cells; when density is high, discard floating cells during medium changes.
4. Seeding density affects cell growth. Low seeding density can lead to slow proliferation and excessive floating cells. Control the passage ratio carefully.
5. Cells are sensitive to temperature and pH. Before passaging or medium changes, equilibrate the medium to room temperature for at least 4 hours. Avoid using medium with altered pH (acidic: yellow; alkaline: purple-red).
6. If single-step dissociation takes too long during passaging, perform dissociation in steps. Avoid forcibly pipetting cells to prevent mechanical damage.
Prev: THP-1 Cell Culture Guide: Common Issues and Solutions
Next: Technical Principles and Procedures for Cell Cryopreservation and Resuscitation





