THP-1 Cell Culture Guide: Common Issues and Solutions
Source: PricellaPublished: 2024-09-06
THP-1 cells are a human monocytic leukemia cell line established by Tsuchiya et al. in 1980. Originating from the blood of a patient with acute monocytic leukemia, these cells have the capacity to differentiate into various types of macrophages and are commonly used in research across multiple fields.
Basic Information of THP-1 Cells
Cell Name | THP-1 |
Cell Alias | THP1; THP 1; THPI; THP-1; Tohoku Hospital Pediatrics-1 |
Species | Human |
Tissue Source | Acute monocytic leukemia, monocytes |
Growth Characteristics | Suspension cells |
Cell Morphology | Monocytes |
Growth Medium | RPMI-1640 + 10%FBS +0.05mM β-mercaptoethanol + 1% P/S |
Culture Conditions | Atmosphere: 95% Air, 5% CO2 |
Recommended Subculture Ratio | 1:2-1:4 |
Freeze medium | 90% FBS + 10% DMSO |
▲ Product Details Cell Line: THP-1 Culture Medium: CM-0233
Characteristics of THP-1 Cells
1. Preference for Acidic Environment
THP-1cells grow faster in a slightly acidic environment. When the culture medium becomes slightly yellow (orange-red), it is suitable for cell growth, and replenishing or partially changing the medium is recommended.
2. Density Dependence
THP-1 cells grow slowly at low densities. It is best to maintain the culture density between 500,000 to 1,000,000 cells/mL. When the density exceeds 2,000,000 cells/mL, subculturing is necessary.
3. High Serum Requirement
Using high-quality serum promotes better growth of THP-1 cells.
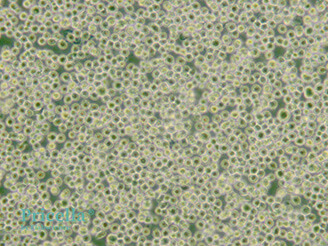
mages of THP-1 Cells Growth
THP-1 Cell Culture Guide: Receiving Cells
1. Room Temperature Cells: Upon receiving THP-1 cells, place them in the incubator for 2-4 hours. Then, centrifuge the entire culture medium (1200 rpm or 250g, 3 minutes), remove the supernatant, resuspend the cells in an appropriate amount of fresh medium, and seed them into new culture flasks at the desired ratio.
2. Frozen Cells: Upon receiving frozen THP-1 cells, store them at -80°C or in liquid nitrogen if not immediately thawing. If thawing, follow the standard thawing procedure.
Tip: Since THP-1 cells exhibit density dependence, placing the culture flask vertically (cap facing up) in the incubator can increase local cell density and promote cell growth. Ensure the cap is ventilated.
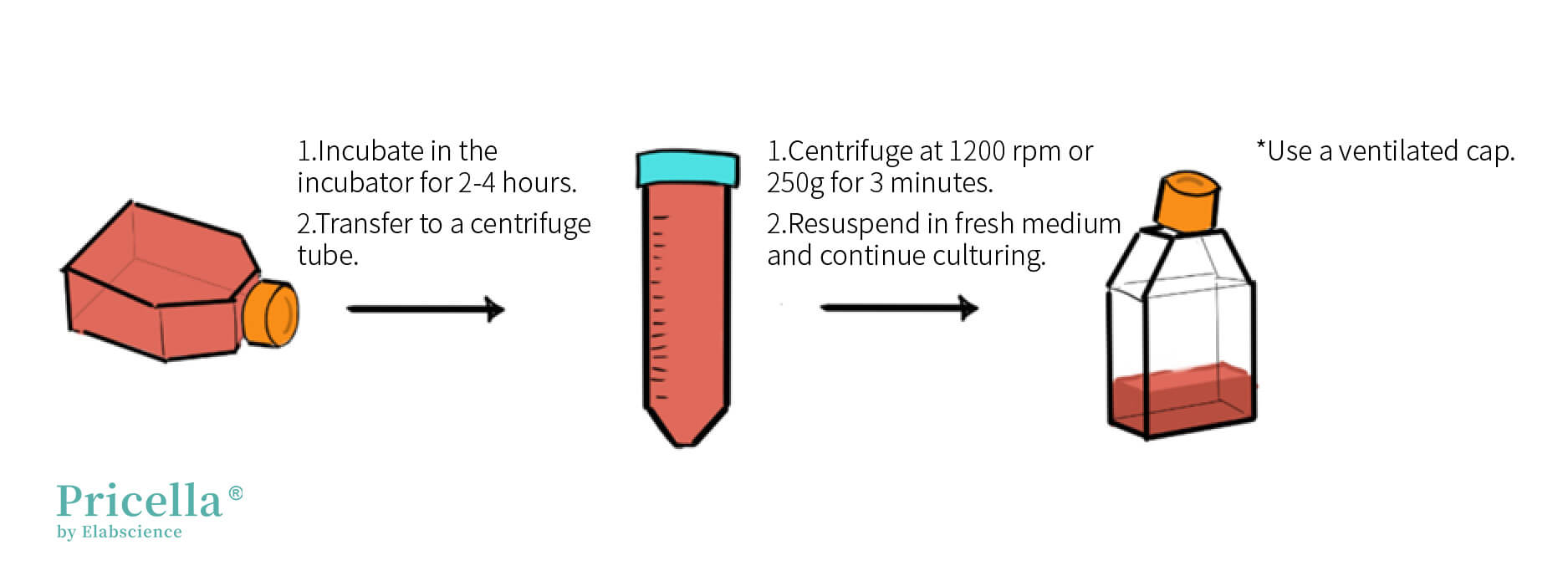
Handling Room Temperature Transportation THP-1 Cells (Illustration)
THP-1 Cell Culture Guide: Medium Change
1. Replenishment Method: When the medium turns yellow, add an appropriate amount (1-2 mL) of fresh medium. After replenishing once or twice, change the medium completely by centrifugation (1200 rpm or 250g for 3 minutes).
2. Partial Medium Change Method: For example, in a T25 flask containing 5 mL of medium, allow the cells to settle (observable by eye), carefully remove 2.5 mL of medium, centrifuge it (1200 rpm or 250g for 3 minutes), and check for cell pellets to avoid cell loss. Add 2.5 mL of fresh medium to the original flask. If cells are present in the centrifuge tube, resuspend them in fresh medium and return them to the original flask.
Tip: If the medium turns very yellow the day after the change, and contamination is not observed under the microscope, it indicates high cell density, requiring subculturing.
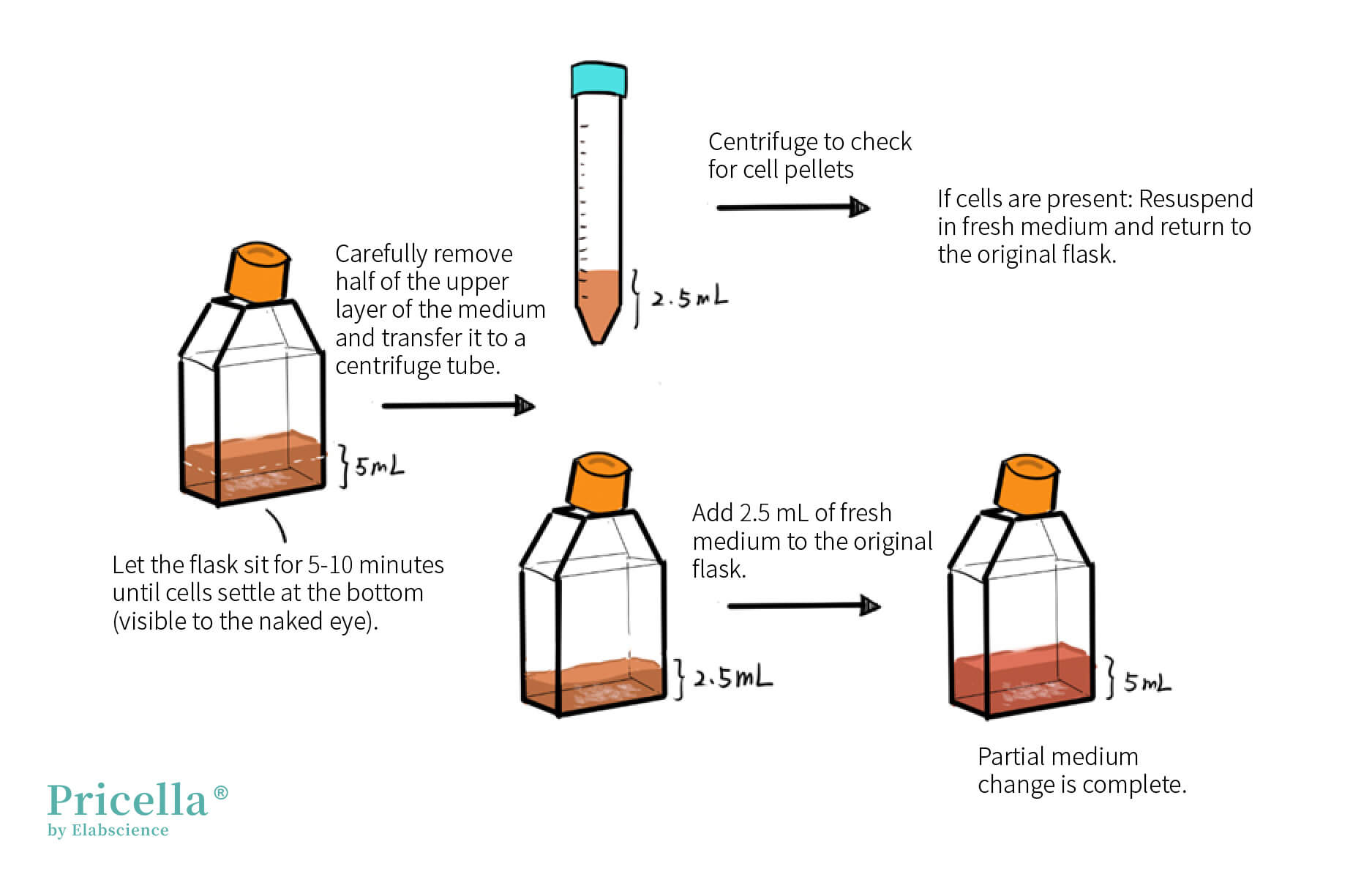
THP-1 Cell Culture Guide: Subculturing
1. Direct Splitting Method: Shake the culture flask to evenly distribute the cells, then split them into two flasks and add an equal amount of medium to each.
2. Centrifugation Method: After centrifugation and counting, seed the cells at a density of 400,000-500,000 cells/mL into T25 flasks with 5 mL of medium. If counting is inconvenient, subculture at a 1:2 ratio.
Tip: When cell debris is abundant, subculturing via centrifugation is recommended, with the speed adjusted appropriately (800-1000 rpm or 160-200g).
Timing for Subculturing THP-1 Cells
THP-1 Cell Culture Guide: Cryopreservation
Step1:Prepare freezing medium in advance.
Step2:Centrifuge cells at 1200 rpm (250g) for 3 minutes.
Step3:Remove the supernatant and resuspend cells in the prepared freezing medium.
Step4:Transfer the cells into cryovials.
Step5:Place the cryovials into a programmed cooling box.
Step6:Store the programmed cooling box at -80°C overnight, then transfer to liquid nitrogen for long-term storage.
Tips:
1. As THP-1 cells are suspension cells and more susceptible to cryopreservation damage, it is advisable to increase the cryopreservation density to approximately 2-3 million cells/mL to improve post-thaw survival rates.
2. If a programmed cooling box is not available, use non-programmed freezing medium (consult the reagent supplier for suitability with THP-1 cells) or manually cool the cells using the following steps: [4°C for 20 min → -20°C for 30 min → -80°C overnight → liquid nitrogen storage].
THP-1 Cell Culture Guide: Thawing
Step1:Preheat a water bath to 37°C.
Step2:Prepare a 15 mL centrifuge tube with approximately 9 mL of complete medium.
Step3:Remove cells from -80°C or liquid nitrogen and place them in a disposable PE glove. Immediately immerse in the water bath and shake vigorously to thaw within 1 minute.
Step4:Wipe the cryovial with alcohol and transfer the thawed cell suspension to the prepared centrifuge tube.
Step5:Centrifuge at 1200 rpm (250g) for 3 minutes.
Step6:Prepare a new T25 culture flask with 5 mL of complete medium.
Step7:Remove the supernatant, resuspend cells in fresh medium, and add to the flask. Place in the incubator.
Tip: THP-1 cells typically require 3-5 days to recover post-thawing. Avoid handling the cells within 48 hours of thawing.
Common Problems and Solutions
Q: What if cells appear with pseudopodia or excessive debris upon receipt?
A: Suspension cells may temporarily change morphology due to transportation, including the appearance of pseudopodia. Normal morphology usually returns within a week through subculturing. Dead cells producing debris during transport can be removed by subculturing and centrifugation.
Q: What to do if cells adhere during culture?
A: A small amount of cell adhesion is normal. Transfer the cell suspension to a new flask. If adhesion exceeds 20%, check the incubator settings, medium composition, and culture vessels for anomalies.
Q: What to do if cells aggregate during culture?
A: A small amount of cell aggregation in a grape-like cluster is normal, especially at low cell densities. Changing the serum brand or increasing the serum ratio (not exceeding 20%) can help resolve aggregation. Do not attempt to disperse aggregated cells manually; they will naturally disperse as density increases.
Q: Is it necessary to add β-mercaptoethanol to the medium?
A: β-mercaptoethanol is a common supplement with antioxidant properties that reduces oxidative stress on THP-1 cells. If cell density is high and needs to be maintained, the proportion of β-mercaptoethanol can be increased (not exceeding 1.5 times the standard amount).
Q: What if it’s difficult to focus or estimate cell density under the microscope?
A: Suspension cells may move with the liquid, making focusing difficult. Let the cells settle for 5-10 minutes, then gently place under the microscope to help focus and estimate cell density.
Prev: Optimizing 22RV1 Cell Growth: Detailed Protocols and Best Practices
Next: How to Address Morphological Changes and Dissociation Issues in bEnd.3 Cells





