LNCaP Clone FGC Cell Culture: A Comprehensive Guide
Source: PricellaPublished: 2024-05-15
The human prostate cancer cell line LNCaP Clone FGC was isolated in 1977 from a fine needle aspiration biopsy of the left clavicular lymph node of a 50-year-old Caucasian male (blood type B+). At the time, the patient had already been diagnosed with metastatic prostate cancer. According to reports, this cell line responds to 5-alpha-dihydrotestosterone (regulating growth and producing acid phosphatase, ACP).
How to Culture LNCaP Clone FGC: Today, Pricella will provide you with a detailed guide.
Important Considerations for Culturing LNCaP Clone FGC
1.Colony Formation: The LNCaP Clone FGC cells do not form a uniform monolayer; instead, they grow in colonies.
2.Acidification and Slow Growth: The culture medium may acidify rapidly due to the cells' metabolic activity, and cell growth is generally slow. Avoid disturbing the cells within 48 hours after passaging to allow them time to settle and grow.
3.Cell Attachment: Standard TC-treated culture flasks or dishes do not support optimal cell attachment, making culturing more challenging. Use Corning's CellBIND® cell culture flasks (Product Number 3289) or culture dishes coated with poly-L-lysine solution (Cat.No.: PB180523) to improve cell adhesion.
4.Cell Recovery During Transportation: During cell transportation, many cells may detach from the culture flask's surface and remain suspended in the medium. Follow the appropriate handling and recovery instructions upon receiving the cells to restore them to normal culture conditions.
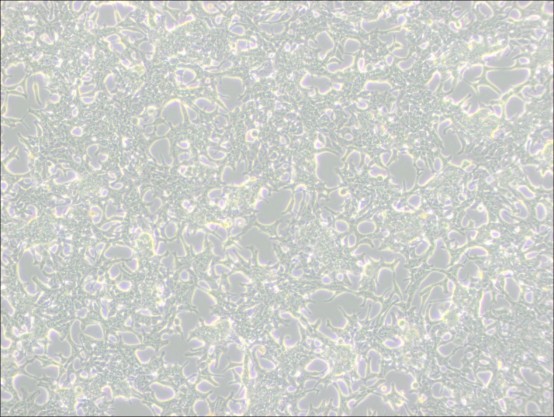
▲ Normal Growth of LNCaP Clone FGC Cells
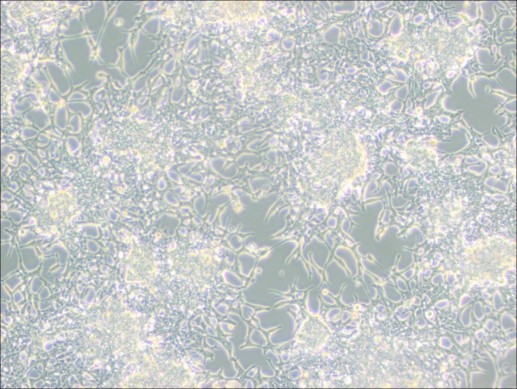
▲ Normal Growth of LNCaP Clone FGC Cells
Handling Process for Received Cells
If, upon receipt, you find that the cells are floating or clumped together, follow these steps to process the cells:
1.Transfer Medium to Centrifuge Tube: Transfer all the culture medium from the culture flask into a sterile centrifuge tube and centrifuge to collect the cells (1200 rpm for 3 minutes).
2.Re-suspend in PBS: Discard the supernatant and re-suspend the cells in PBS. Collect the cells into one centrifuge tube, and gently shake the tube to mix the cells. Centrifuge again (1200 rpm for 3 minutes).
3.Digest with Trypsin: Discard the supernatant and add about 1 mL of 0.25% trypsin to re-suspend the cells and mix gently. Do not pipette the cells up and down. Place the tube in an incubator to digest the cells for approximately 3 minutes.
4.Terminate Digestion: Once digestion is complete, gently pipette the cell suspension to break up any clumps. Add 5 mL of serum-containing culture medium to terminate digestion, and then centrifuge (1200 rpm for 3 minutes).
5.Re-suspend Cells and Seed: Discard the supernatant and add 5-7 mL of appropriate complete growth culture medium to the cells. Gently pipette the cells up and down to ensure even mixing. Seed the cells in a sterile T25 flask (the culture vessel should be pre-conditioned with the corresponding culture medium).

▲ LNCaP Clone FGC Cells Arriving Floating
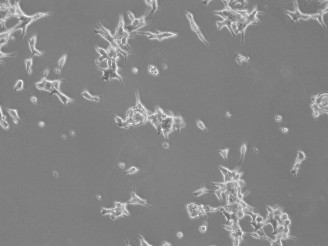
▲ Low Seeding Density of LNCaP Clone FGC Cells
Steps for Passaging Cells
1.Remove Old Culture Medium: Aspirate the existing culture medium from the flask.
2.Rinse with PBS: Add approximately 2 mL of PBS to gently rinse the cells in the culture flask. Aspirate the PBS and discard it.
3.Add Trypsin: Add about 1 mL of trypsin to the culture flask and gently swirl it around to coat all the cells.
4.Incubate for Digestion: Place the flask in the incubator for approximately 5 minutes for cell digestion. Under the microscope, you should observe that the cells in the cell clumps begin to separate and become round as they naturally detach. Do not tap the flask during this process.
5.Terminate Digestion: Add 3 mL of serum-containing culture medium to terminate the trypsin digestion. Gently pipette the cell suspension up and down to ensure the cells are mostly in single-cell form. Observe under a microscope to verify the cells are properly dispersed.
6.Collect and Centrifuge Cells: Collect the cell suspension and centrifuge at 1200 rpm for 3 minutes. After centrifugation, remove the supernatant and discard it.
7.Re-suspend and Seed Cells: Add fresh culture medium to the cell pellet and pipette gently to mix and re-suspend the cells. Seed the cells in new culture flasks as needed, and add sufficient fresh culture medium. Loosen the cap of the flask slightly or use a vented cap for culture.
8.Check Incubator Settings: Ensure that the incubator's carbon dioxide levels, temperature, and water tray are set appropriately.
Passaging Ratio and Frequency
- Passaging Ratio: It is recommended to passage the cells at a ratio of 1:2 to 1:4.
- Frequency: Passage the cells approximately every 4 to 5 days
Medium Replacement
- Remove the old culture medium, add fresh culture medium along the side of the culture flask.
- Replace the medium every 3 days.
Freezing Steps
1.Prepare Freezing Medium: The recommended ratio is 55% RPMI-1640 medium, 40% serum, and 5% DMSO.
2.DMSO Handling: When preparing the freezing medium, be cautious as DMSO generates heat during preparation. Allow the freezing medium to cool before using it to avoid cell damage.
3.Prepare Cell Suspension: After digesting the cells, stop digestion using fresh culture medium and create a cell suspension.
4.Centrifuge Cells: Centrifuge the cell suspension at 1200 rpm for 3 minutes. Remove the supernatant carefully to minimize residual fluid.
5.Add Freezing Medium: Add the prepared freezing medium to the cell pellet and resuspend. For a T25 flask, one full flask can be frozen as one vial. Alternatively, count the cells and freeze at a density of 2-5 × 10^6 cells per vial.
6.Freeze Slowly: Transfer the vials to a controlled-rate freezing box and store them in a -80°C freezer overnight.
7.Transfer to Liquid Nitrogen: The next day, transfer the vials from the -80°C freezer to liquid nitrogen storage for long-term preservation.
Thawing Steps
1.Prepare Warm Water Bath: Preheat a water bath to 37°C. Prepare a sterile centrifuge tube with 9 mL of sterile culture medium.
2.Thaw the Cells: Remove the LNCaP Clone FGC cell vial from the liquid nitrogen storage and quickly place it in the water bath while wearing clean, disposable gloves. Shake the vial gently to accelerate thawing, aiming to thaw the contents within one minute.
3.Centrifuge the Cells: In a sterile environment, add the thawed cell suspension to the prepared centrifuge tube containing fresh medium. Centrifuge the mixture at 1200 rpm for 3 minutes. After centrifugation, remove the supernatant.
4.Resuspend in Fresh Medium: Resuspend the cells in an appropriate amount of complete culture medium. Seed the cells into a sterile culture flask or dish and add additional culture medium.
5.Rapid Processing: Thawing should be done quickly. Avoid leaving the thawed cells at room temperature for too long. After thawing, promptly centrifuge the cells to remove DMSO and resuspend them in fresh medium.
Prev: How to Successfully Culture SH-SY5Y Cells: Key Details to Consider





