Adhesion Issues? Tips for Culturing TM3 and TM4 Cells
Source: PricellaPublished: 2024-05-28
TM3 and TM4 cells are derived from mouse testicular tissue, and although their culture conditions are basically the same, their cellular characteristics differ.
TM3 cells (mouse testicular interstitial cells) increase cyclic adenosine monophosphate (cAMP) production in response to luteinizing hormone (LH) but do not respond to follicle-stimulating hormone (FSH). In the presence of LH, TM3 cells can metabolize cholesterol. The duration of the TM3 cells' response to LH is related to the serum batch.
TM4 cells (normal mouse testicular sertoli cells) increase cAMP production in response to FSH but do not respond to LH. Compared to primary sertoli cells, TM4 cells have a reduced responsiveness to FSH. TM4 cells have a low constitutive production of plasminogen activator, but this can be stimulated by FSH and significantly increased by retinoic acid. TM4 cells produce retinol-binding protein, tissue plasminogen activator, and transferrin. TM4 cells express FSH receptors, androgen receptors, and progesterone receptors and are negative for ectromelia virus (mousepox).
Basic Information
Growth Characteristics: Adherent cells
Cell Morphology: Epithelial-like cells
Growth Medium: DMEM/F12 + 5% HS + 2.5% FBS + 1% P/S
Recommended Subculture Ratio: 1:3-1:4
Recommended Medium Change Frequency: 2-3 times per week
Cryopreservation Conditions: 55% basal medium + 40% FBS + 5% DMSO
Culture Conditions
Gas Phase: Air, 95%; CO₂, 5%
Temperature: 37°C
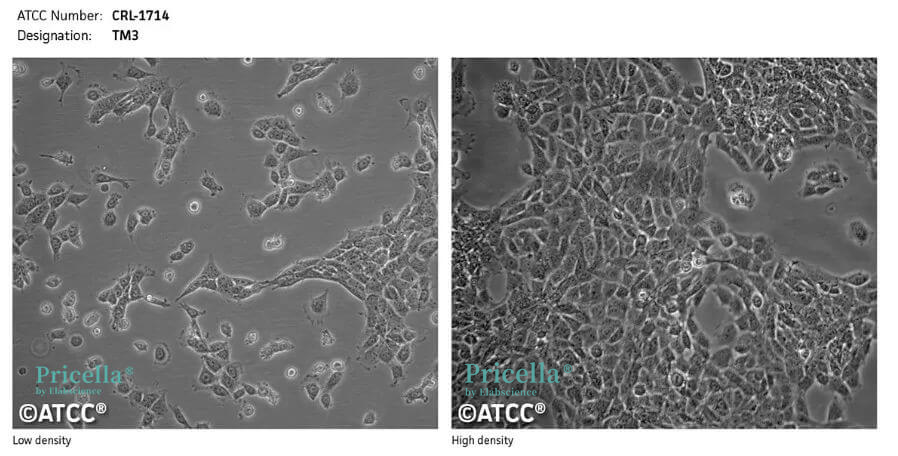
Figure 1. Image of TM3 cells under normal growth conditions.
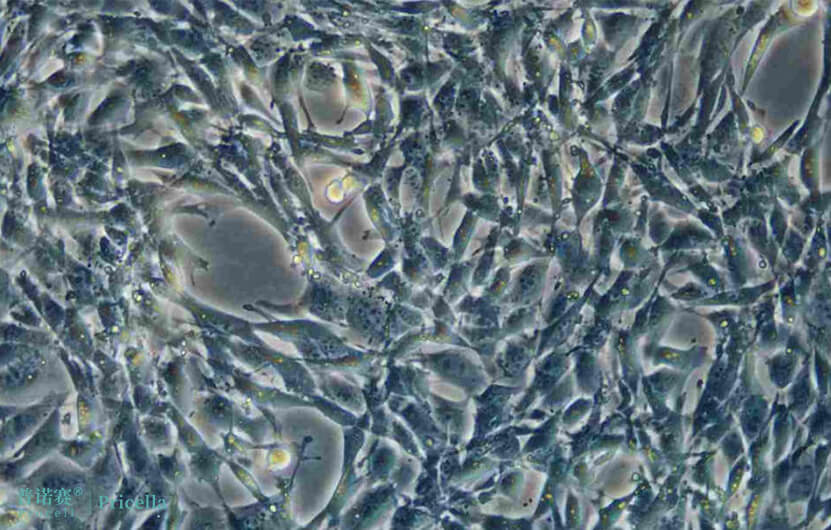
Figure 2. Image of TM4 cells under normal growth conditions.
TM3 and TM4 cells adhere loosely. Due to transport stress, cells may detach and float upon arrival. What should be done in such cases?
If you observe large cell clumps detaching upon arrival of TM3 and TM4 cells, do not worry. Follow these steps to ensure normal cell growth:
1 Transfer all the medium from the culture flask into a sterile centrifuge tube and collect the cells by centrifugation (1200 rpm for 3 minutes).
2 Remove the supernatant, resuspend the cells in PBS, and collect the cells into a single centrifuge tube. Mix the PBS by gently shaking the centrifuge tube, then centrifuge again (1200 rpm for 3 minutes).
3 Remove the supernatant, add approximately 1 mL of 0.25% trypsin to resuspend the cells, and mix by gently shaking the centrifuge tube (do not pipette up and down). Place the tube in an incubator to digest the cells.
4 After 2-3 minutes of digestion, gently pipette the cell suspension to disperse the cell clumps. Add 5 mL of medium containing serum to stop the digestion, and centrifuge (1200 rpm for 3 minutes).
5 Remove the supernatant, add approximately 5 mL of the complete medium, and mix well. Seed the cells into a sterile container.
Tips:
At this point, you can observe the TM3 and TM4 cells under a microscope to see if they are evenly dispersed as single cells. If there are still small cell clumps with 3-5 cells, there is no need to re-digest. Let them adhere and grow stably before they naturally disperse.
After treating the cells following the above steps, they will generally reattach by the next day.
Perfectly solving the issue of receiving cells is just one part of successfully culturing TM3 and TM4 cells. There are many other details to pay attention to during the cell culture process:
- Handle the cells gently during the culture process.
- After digesting for 1-2 minutes, stop the digestion when you see the cells in the middle of the cell clumps visibly separate and become rounded under the microscope. Do not tap the culture flask during the entire process.
- TM3 and TM4 cells grow rapidly and adhere loosely. Once confluent, they will form sheets and float, so be sure to passage them timely.
- There may be cell clumping the day after passaging, but they will usually spread out by the third day.
- Do not seed cells at too high a density; ensure at least 2 days of growth space.
- Pre-warm the medium before changing it.
Prev: LNCaP Clone FGC Cell Culture: A Comprehensive Guide
Next: Strategies for Passaging and Culturing H22 Hepatocellular Carcinoma Cells from Ascitic Fluid





