How to Successfully Culture SH-SY5Y Cells: Key Details to Consider
Source: PricellaPublished: 2024-05-10
The SH-SY5Y cell line was established in 1970 as a subline derived from the SK-N-SH neuroblastoma cell line in bone metastasis through three rounds of cloning (SK-N-SH → SH-SY → SH-SY5 → SH-SY5Y). Today, SH-SY5Y cells are one of the most commonly used cell lines in neuroscience research.
However, many people encounter various issues during the culturing of SH-SY5Y cells. Today, Pricella is here to help answer all your questions.
SH-SY5Y Cell Basic Information
| Cell Name | SH-SY5Y (human neuroblastoma cell) |
| Cell Aliases | SHSY5Y; SHSY-5Y; SH-Sy5y; SK-SH-SY5Y; SY5Y |
| Species and Source | Human (female, 4 years old), brain neuroblastoma with bone marrow metastasis |
| Growth Characteristics | Semi-adherent and semi-suspension |
| Growth Morphology | Epithelial-like |
| Growth Medium | MEM/F12+15% FBS+1% P/S |
| Culture Conditions | Atmosphere: 95% air, 5% CO2; Temperature: 37°C |
| Recommended Passage Ratio | 1:3-1:4 |
| Recommended Media Change Frequency | Every 48 hours |
| Cryopreservation Solution | 55% MEM/F12+40% FBS+5% DMSO |
Common Issues and Solutions in SH-SY5Y Cell Culture
Receiving Cells
During room temperature transport, cells may experience shaking and temperature fluctuations, resulting in cell shrinkage, aggregation, or detachment. Do not panic when faced with such situations. With proper handling, cells can return to normal growth.
Cell Shrinkage Upon Receiving
Once you receive the cells at room temperature, place them in a cell culture incubator and let them sit for 2–4 hours. If the cells haven't spread out after 4 hours, you can leave them to rest overnight as long as the density is moderate. Before doing so, remove some of the culture medium, leaving 5–10 mL in the flask, and loosen the cap.
Cell Aggregation Upon Receiving
Once you receive the cells at room temperature, start by placing them in a cell culture incubator for 2–4 hours to let them stabilize. Then, passage them using 0.25% trypsin with EDTA, allowing them to digest for about 1–3 minutes
Cell Detachment Upon Receiving
After receiving cells at room temperature, first place the cells in a cell culture incubator for 2–4 hours to stabilize them before handling. Detached cells usually form floating aggregates; collect the floating cell clusters by centrifugation, and after trypsin digestion in a centrifuge tube, reseed them onto a culture plate.
Combine the cells that were digested from adherent cultures using conventional methods with the treated detached cells, mix well, and seed onto a culture plate.
Steps for Handling SH-SY5Y Cell Detachment:
1. Centrifuge the cells at 1200 rpm (approximately 250g) for 3 minutes to collect the cells and remove the old culture medium.
2. Resuspend the cells in PBS, then centrifuge them again at 1200 rpm (approximately 250g) for 3 minutes to remove the PBS.
3. Add approximately 1 mL of 0.25% trypsin to resuspend the cells, pipette up and down 1-2 times to resuspend the pellet.
4. Place the cells in a cell culture incubator for approximately 1-3 minutes.
5. Gently pipette the cell suspension to disperse the cell aggregates.
6. Quickly add 3-5 mL of culture medium containing serum to stop the digestion and mix well.
7. Centrifuge the cells at 1200 rpm (approximately 250g) for 3 minutes to remove the trypsin.
8. Finally, add fresh culture medium and transfer the cells into an appropriate sterile culture vessel at a suitable dilution ratio.
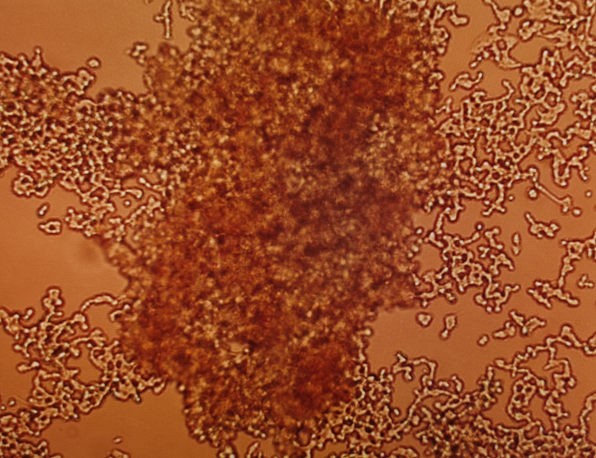
Figure 1. SH-SY5Y Cell Shrinkage and Detachment Upon Receiving
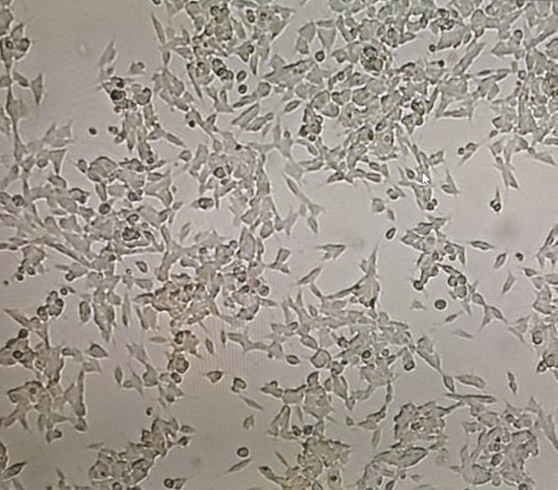
Figure 2. Third Day After Handling SH-SY5Y Cells
SH-SY5Y Cell Culturing
SH-SY5Y cells grow slowly. During the cell culture process, there are a few points to be mindful of.
Can DMEM/F12 be used?
Both MEM/F12 and DMEM/F12 can be used to culture SH-SY5Y cells, and there is literature support for both. However, there may be slight differences in the cell morphology obtained using these two media. Additionally, using high-quality fetal bovine serum can greatly improve cell growth.
Culture Medium Tends to Turn Yellow
Because of the neuroblastoma characteristics of SH-SY5Y cells, they tend to grow in clusters and have a high saturation density. Sometimes the density may not seem high under the microscope, but the actual density of the cell clusters can be quite high, causing the culture medium to turn yellow quickly. In these cases, it's important to change the medium promptly.
Abnormally Slow Growth
If SH-SY5Y cells cannot be passaged within a week, the cells can be digested and seeded into a smaller vessel to artificially increase cell density, thereby promoting cell growth. Additionally, when passaging the cells, be mindful of not using too low a seeding density.
Passaging Considerations
Continuous cell digestion is not recommended, as it can harm the cells. When passaging, keep digestion time short, usually around 1–3 minutes. If the cells detach too quickly, dilute the trypsin with PBS. When pipetting the cells, use gentle movements to avoid causing physical stress.
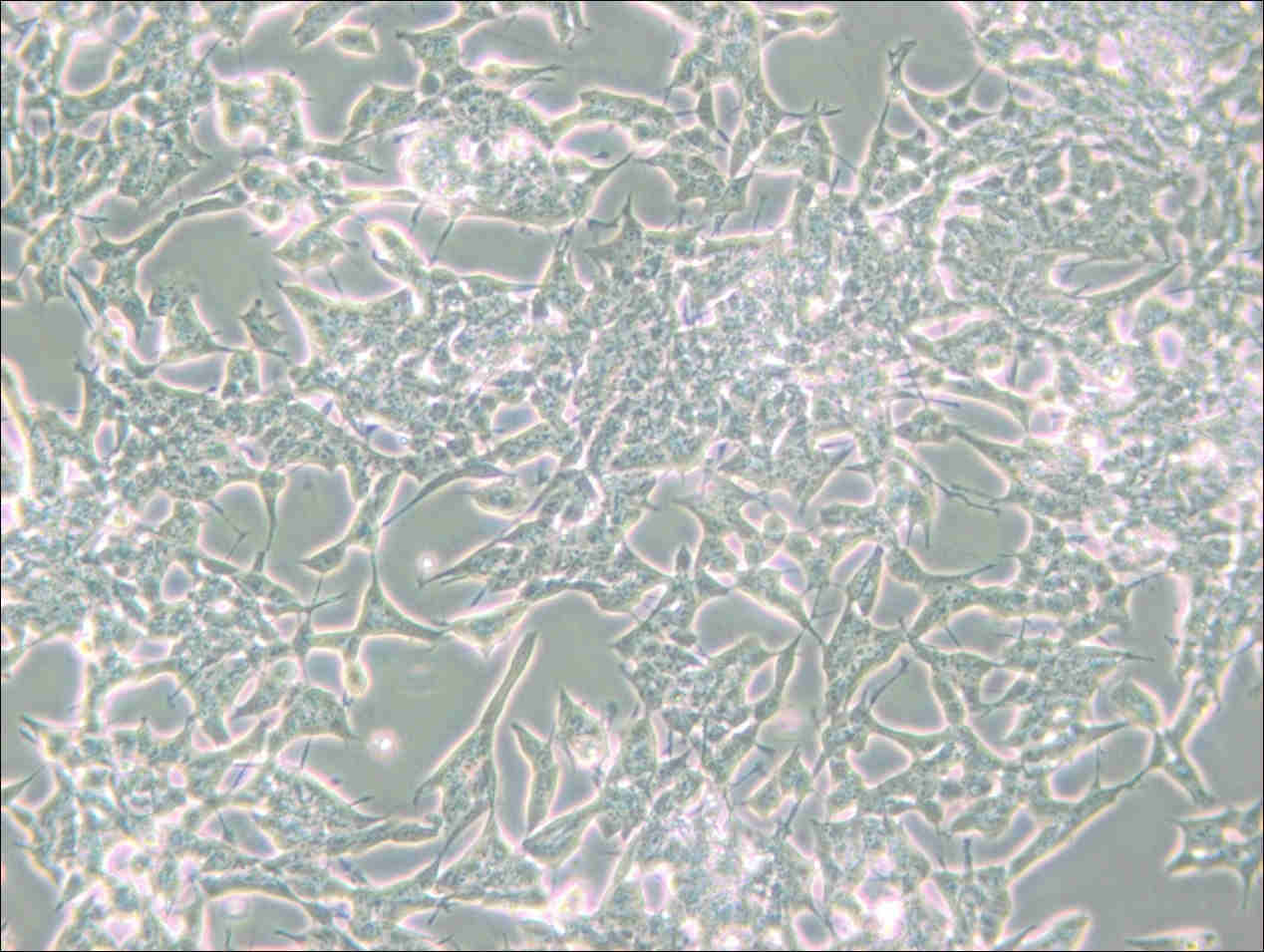
Figure 3. Photos of Normal SH-SY5Y Cell Growth
Morphology
During SH-SY5Y cell culture, both adherent and suspension cells may be present. Adherent cells resemble epithelial cells, with short extensions and a tendency to grow in clusters.
Excessive Suspension Cells: It is normal for SH-SY5Y cells to have a few suspension cells during growth. However, if there are large numbers of suspension cells, it should raise concerns.
Handling Method: During media changes, centrifuge and collect the suspension cells. Resuspend the cells in fresh culture medium and reseed them back into the original flask or dish. When the growth state of the adherent cells is good and the density is moderate, the suspension cells can be discarded.
Severe Aggregation: SH-SY5Y cells are very sensitive and may form aggregates easily when exposed to temperature, chemical, or physical stress. If there are only small amounts of aggregation visible in one field of view, no special handling is needed. However, if there is severe aggregation with cells barely spreading out, follow the methods below for special handling.
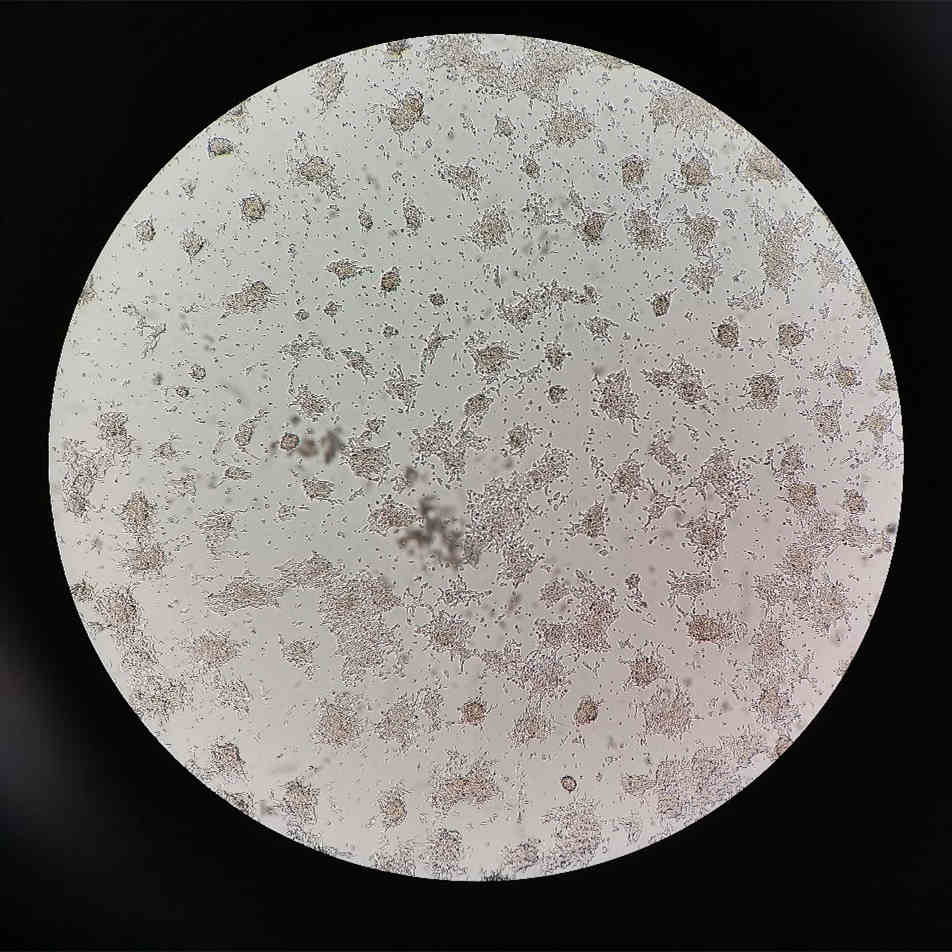
Figure 4. Severely Aggregated SH-SY5Y Cells
Handling Methods
Method 1: Seed at a low density, digest the cells with 0.125% low-concentration trypsin (no longer than 5 minutes), and passage at a 1:6 ratio. Use a new culture vessel and extend the media change interval to every 3 days to prevent cell detachment.
Method 2: Use culture vessels coated with poly-L-lysine to speed up cell attachment and reduce the likelihood of cell aggregation.
Method 3: Reseed the cells, use freshly prepared culture medium, and increase the serum proportion (up to a maximum of 20%).
Method 4: When seeding, reduce the amount of culture medium (e.g., use 3 mL for T25 flasks) to accelerate cell attachment. After the cells attach (approximately 8–12 hours), add more culture medium.
Methods to Prevent Aggregation
- Control the cell density to not exceed 80%; passage the cells when the density reaches or exceeds 80%. Avoid vigorous pipetting during passaging to prevent mechanical stress on the cells.
- Use high-quality culture medium and fetal bovine serum to minimize the exposure of cells to harmful substances such as endotoxins.
- Avoid frequently removing cells from the incubator. In cooler climates, use heating to maintain the temperature of the cell culture room. Preheat PBS and culture medium to 37°C before use to avoid temperature shock to the cells.





