Decoding Astrocytes: Essential Techniques for Primary Cell Isolation
Source: PricellaPublished: 2025-05-29
Astrocytes are crucial supportive cells in the brain, playing diverse biological roles. Given their pivotal involvement in neurodegenerative diseases, neural injury, and neurodevelopment, astrocytes have emerged as ideal targets for therapeutic interventions in neurological disorders.
In this article, we will introduce the background of astrocytes and the methods for primary astrocyte isolation, to help you quickly understand astrocytes and successfully carry out related experiments.
Cell Characteristics
Astrocytes are essential supportive cells in the brain that perform diverse biological functions. Their critical roles in neurodegenerative diseases, neural injury, and neurodevelopment make them ideal targets for therapeutic interventions in neurological disorders.
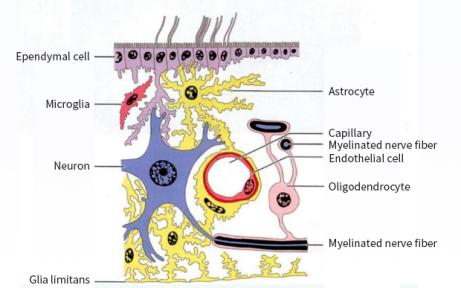
Fig.1 Schematic diagram of the relationship between glial cells, neurons, and capillaries in the central nervous system (image sourced from the internet)
Biological function
1 Neuronal Support Function: Astrocytes interact with neurons through tripartite synapses, regulating synaptic transmission and modulating the activities of vascular endothelial cells, pericytes, and the basement membrane. This helps maintain neuronal metabolism and blood flow[1].
2 Neuroprotection Paradox: Under pathological conditions, astrocytes can form glial scars. This limits inflammation but also restricts axonal regeneration. Additionally, they secrete neurotoxic lipids and factors that promote neuronal death or induce apoptosis.
3 Regulation of Neuroplasticity: Mature astrocytes influence the reopening of the critical period in the visual system by regulating neuroplasticity, highlighting their significance in this process.
4 Hub of Neuroinflammation: Astrocytes play a central role in neuroinflammation. They secrete inflammatory factors and neurotoxic substances, which contribute to the development of neurodegenerative diseases such as Alzheimer's disease (AD)[2,3].
Markers and Functional Analysis
1 GFAP (Glial Fibrillary Acidic Protein): A characteristic marker of astrocytes, its expression level is positively correlated with cell activation status. It is widely used for detecting and studying the distribution and function of astrocytes [3-4].
2 S100B: Highly specific to gray matter astrocytes. It is used to distinguish astrocytes in gray and white matter and also serves as a biomarker for astrocyte injury.
3 SOX10: Expressed in astrocyte precursors and mature astrocytes. It is commonly used as a marker for studying astrocyte differentiation and development.
4 GLAST and GLT-1: These two transporters are primarily responsible for glutamate clearance and are indispensable functional markers of astrocytes in neuronal metabolism.
5 AQP4 (Aquaporin-4): Highly expressed in the perivascular endfeet of astrocytes. It is involved in brain edema and the regulation of brain water homeostasis.
Research Applications
1 Learning and Memory Mechanisms: Astrocytes regulate the synaptic activity of memory engram neurons by releasing D-serine, which enhances NMDA receptor function and promotes memory formation.
2 Neurodegenerative Diseases: In Alzheimer's disease (AD) and Parkinson's disease (PD), astrocytes release inflammatory factors and neurotoxic substances, affecting neuronal survival and are correlated with Aβ plaque formation and dopaminergic neuron function. Specifically, in AD, elevated levels of GFAP and S100B in astrocytes indicate neuroinflammation and neurodegenerative changes.
3 Spinal Cord Injury: Astrocyte proliferation and glial scar formation are typical responses following injury, with significant increases in markers such as GFAP and PCNA.
4 Epilepsy: Abnormal activation of astrocytes is associated with temporal lobe epilepsy, and changes in GFAP and SOX10 expression suggest a role for astrocytes in the pathogenesis of epilepsy.
5 Neurotoxicity Studies: Astrocytes are utilized in screening drugs and environmental toxins to assess their neurotoxicity.
6 Neuroregeneration and Repair: Following spinal cord injury and stroke, astrocytes form glial scars and secrete neurotrophic factors, promoting repair and regeneration.
7 Neuronal Maturation and Brain Organoids: Astrocyte-secreted factors promote neuronal maturation and support the construction of brain organoids, which mimic real brain structures.
The Primary Cells Isolation and Culture
The primary cells isolation and culture of astrocytes are a crucial technique for studying their physiological and pathological mechanisms. This experiment uses Sprague-Dawley rats (1-3 days old) as subjects to isolate astrocytes from cerebral cortical tissue. The specific steps are as follows:
Specimen sampling
1 Take a newborn rat, decapitate it, and make a midline incision in the skull to remove the intact brain, which is then placed in pre-cooled PBS.
2 Use micro-scissors and micro-forceps to dissect the cerebral cortex.
3 Use micro-forceps under a dissecting microscope to peel off the meninges of the cerebral cortex, or place the cortical tissue on sterile filter paper and roll it back and forth twice to remove the meninges.
4 Collect the processed cortical tissue and cut it into small pieces of 1-3 mm³.
Tissue Processing and Digestion
1 Add 3-5 times the tissue volume of digestion enzyme. Incubate at 37℃ with shaking in a water bath for 10 min.
2 Add FBS to stop the digestion. Pipette repeatedly until the tissue is dispersed.
3 Filter sequentially through 100-mesh and 200-mesh cell strainers to remove large tissue fragments and brain microvascular tissue.
4 Collect the filtrate and centrifuge at 1200 rpm for 5 min to obtain the cell pellet.
5 Resuspend the cells in complete medium and seed them in T25 flasks at a density of 5-10×105 cells per flask.
Cell Isolation
After enzymatic digestion of the cerebral cortex tissue, it is directly plated for culture, resulting in a mixed cell population that includes astrocytes, microglia, oligodendrocytes, and neurons. Among these, astrocytes account for approximately 48%, microglia for about 11%, neurons for around 40%, and the remaining 1% consists of other cell types.
After 24 h of culture, the medium is replaced to remove some of the neuronal cells. Continued culture for about one week yields a higher purity of astrocytes.
Purification Method 1
The cells are digested and passaged to remove microglia (microglia cannot proliferate and are difficult to digest; multiple passages can purify the astrocytes).
Purification Method 2
The culture flask is sealed and placed on a shaker at 200 rpm for 48 h to remove floating microglia cells, leaving behind the adherent high-purity astrocytes.
Cell Identification
The primary isolated rat astrocytes are identified by GFAP immunofluorescence, achieving a purity of up to 90% (Fig. 2). Rat neural astrocytes cultured for 10 d exhibit elongated processes with extensive branching and widespread extensions, small cell bodies, and a network-forming star-shaped structure, which are typical characteristics of astrocytes (Fig. 3).
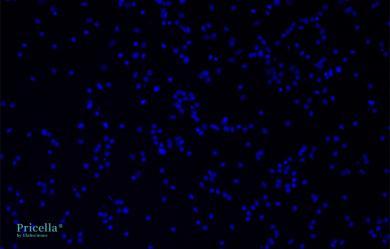
DAPI
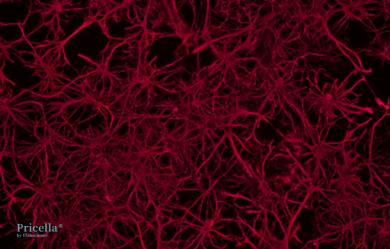
GFAP
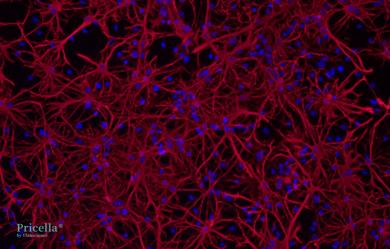
Merge
Fig. 2 Immunofluorescence Identification of Rat Neural Astrocytes, 200×
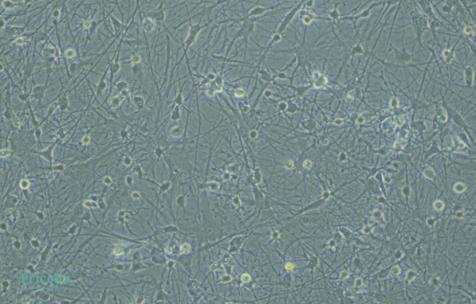
Fig. 3 Rat Neural Astrocytes, 200× (Left: Cultured for 3 d; Right: Cultured for 10 d)
Precautions
All instruments, consumables, and reagents must be guaranteed to be sterile and operated in a sterile environment.
The entire operation must be maintained at a low temperature of 4℃ and performed on an ice box.
The meninges must be thoroughly cleaned; otherwise, fibroblasts, microvessels, and other contaminating cells may be introduced.
The operation time should be minimized. Prolonged operation may lead to tissue ischemia, death, or autolysis, thereby affecting cell viability.
During digestion, be cautious of DNA clumps produced by cell lysis. These need to be treated with DNase; otherwise, they may cause cell adhesion, clog the filter mesh, and significantly reduce cell yield.
References
[1] Tsujioka H, Yamashita T. Utilization of ethanolamine phosphate phospholyase as a unique astrocytic marker. Frontiers in Cellular Neuroscience. 2023 January 30; 17: 1097512.
[2] Kong Y, Cao L, Wang J, Zhuang J. In vivo reactive astrocyte imaging using [18F]SMBT-1 in tauopathy and familial Alzheimer's disease mouse models: A multi-tracer study. Journal of the Neurological Sciences. 2024 July 15; 462: 123079.
[3] Barclay WE, Aggarwal N, Deerhake ME. The AIM2 inflammasome is activated in astrocytes during the late phase of EAE. JCI Insight. 2022 April 22; 7 (8): e155563.
[4] Tsujioka H, Yamashita T. Utilization of ethanolamine phosphate phospholyase as a unique astrocytic marker. Frontiers in Cellular Neuroscience. 2023 January 30; 17: 1097512.
[5] Sidoryk-Wegrzynowicz M, Gerber YN, Ries M. Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta Neuropathologica Communications. 2017 Nov 29;5(1):89.
[6] Li G, Cao Y, Shen F. Mdivi-1 inhibits astrocyte activation and astroglial scar formation and enhances axonal regeneration after spinal cord injury in rats. Frontiers in Cellular Neuroscience. 2016 October 19; 10: 241.
[7]Pai B, Tome-Garcia J, Cheng WS. High-resolution transcriptomics informs glial pathology in human temporal lobe epilepsy. Acta Neuropathologica Communications. 2022 October 23; 10 (1): 149.
Next: Optimizing Cell Transfection Conditions in "Four Steps": Say Goodbye to Inefficiency





