Optimizing Cell Transfection Conditions in "Four Steps": Say Goodbye to Inefficiency
Source: PricellaPublished: 2025-06-03
Cell transfection is a widely used laboratory technique that involves introducing exogenous nucleic acids (such as DNA, mRNA, siRNA, and so on) into recipient cells. It plays a crucial role in gene function research, disease model construction, protein expression, and drug development.
However, due to the varying physiological characteristics of cells from different sources, their sensitivity to transfection conditions differs significantly, leading to inconsistent transfection efficiency. Therefore, effectively improving this efficiency has become an important challenge for researchers.
In this article, we will share four key optimization steps to help you easily enhance transfection efficiency.
Common cell transfection methods include chemical, physical, and viral vector methods[1].
Chemical methods include liposome transfection, DEAE-dextran, and cationic polymer transfection. The principle involves using transfection reagents (such as cationic liposomes, DEAE-dextran, cationic polymers, and so on) to form complexes with negatively charged nucleic acids, which then enter cells through endocytosis, achieving cell transfection.
Chemical transfection methods are characterized by their simplicity and broad applicability but also carry a degree of cytotoxicity[2-4]. Therefore, selecting the appropriate transfection reagent is crucial for achieving high transfection efficiency, especially for different cell types.
Physical methods include microinjection and electroporation[5]. Among these, electroporation uses high-voltage electric pulses to create transient pores in the cell membrane, allowing nucleic acids and other biomolecules to enter the cells.
This method offers high transfection efficiency but can causes significant cell damage and result in high cell mortality rates.
Viral Vector Methods
Viral vector methods, such as lentivirus, adenovirus, and retrovirus, are used. Notably, lentivirus and retrovirus can integrate their genomes into the host cell, enabling stable and long-term gene expression[6]. However, viral vector methods posecytotoxic and potential biosafety risks, requiring strict operational protocols.
Selecting the appropriate transfection method based on cell type and experimental objectives is crucial. For most adherent cells, liposome transfection is a commonly used and relatively gentle method. For primary cells or difficult-to-transfect suspension cells, electroporation or viral vector transfection may be more suitable.
Cell density is one of the critical factors influencing transfection efficiency.
Generally, adherent cells exhibit better transfection results at 70%-90% confluency. However, for certain specific cell lines or transfection methods, it may be necessary to adjust the cell density.
It is recommended to seed cells at multiple density levels one day before transfection to achieve different confluency levels at the time of transfection. After transfection, evaluate the transfection efficiency and cell condition of each group through experiments to determine the optimal cell density range.
Taking lipofection as an example, the ratio of lipofection reagent to target material significantly affects transfection efficiency and cytotoxicity.
To optimize transfection results, it is recommended to add an equal amount of cell suspension to each well when cells are digested and seeded into the plate, ensuring consistent cell density during transfection.
According to the recommended ratio in the transfection reagent manual, set up gradients, such as transfection reagent to target material ratios of 1:1, 2:1, and 3:1, to prepare transfection complexes of different concentrations, which are then added to the corresponding wells. Each ratio should be set up with three replicate wells to ensure data reliability.
After incubating the culture plate in the cell incubator for a certain period, observe transfection efficiency using a fluorescence microscope or flow cytometry, and detect cell viability using the CCK-8 assay. Based on the experimental results, select the optimal ratio with high transfection efficiency and low cytotoxicity for subsequent experiments.
Optimizing Incubation Time
The incubation time of cells with transfection complexes is a crucial factor influencing transfection efficiency.
Insufficient incubation may prevent the transfection complexes from adequately entering the cells, leading to low transfection efficiency. Conversely, prolonged incubation can increase the cytotoxicity of the transfection reagent, adversely affecting cell health.
To optimize transfection outcomes, it is recommended to replace the medium at multiple time points post-transfection (e.g., 6 h, 12 h, 24 h) and monitor changes in transfection efficiency and cytotoxicity. For cells that are particularly sensitive to transfection reagents, reducing the incubation time may help minimize cell damage while maintaining high transfection efficiency. Determining the optimal incubation time through experimentation is essential to achieve a balance between transfection efficiency and cell viability.
After completing a series of optimization experiments, it is necessary to systematically summarize the results. Compare data on transfection efficiency and cell survival rates under different conditions, and analyze the impact of various parameters on transfection outcomes.
Based on the experimental results, develop a strategy for optimizing transfection conditions tailored to specific cell lines or experimental systems, providing a reference for subsequent experiments.
Optimization of Transfection Conditions for Hep G2 Cells
Experimental Objective
To determine the optimal transfection conditions for human hepatocellular carcinoma cells (Hep G2), this study introduced the enhanced green fluorochrome protein gene (EGFP) into Hep G2 cells under different transfection conditions and observed its expression.
Preliminary Experiment
In the preliminary experiment, we selected a conventional transfection reagent and strictly followed the instructions. However, the transfection efficiency was very low, with less than 10% of the cells expressing the fluorochrome protein.
Optimization Strategy
To improve transfection efficiency, we optimized the following aspects:
Cell Culture Conditions: Selected a medium suitable for the growth of Hep G2 cells.
Transfection Reagent Screening: Tested multiple transfection reagents and selected the one with the best transfection performance, further optimizing its transfection protocol.
Quality and Concentration of Plasmid: Used high-quality plasmid extraction kits and reagents to ensure the purity and integrity of the plasmid. Through concentration gradient experiments, we tested different ratios (1:1, 2:1, 3:1, 4:1, 5:1) of transfection reagent (μL) to plasmid (μg) and observed the expression effect of EGFP in Hep G2 cells under different ratios. The experimental results showed that the optimal ratio of transfection reagent to plasmid was 2:1 (Fig. 1).
Incubation Time and Temperature of Transfection Complex: Set different incubation times and temperature gradients to observe their impact on transfection efficiency. The experiment found that the transfection effect was optimal when the transfection complex was incubated at room temperature (20-25℃) for 20 min.
Experimental Results
After a series of optimization experiments, the transfection efficiency of Hep G2 significantly improved. More than 60% of the cells expressed EGFP, achieving high-efficiency expression of EGFP in Hep G2 cells (Fig. 2).
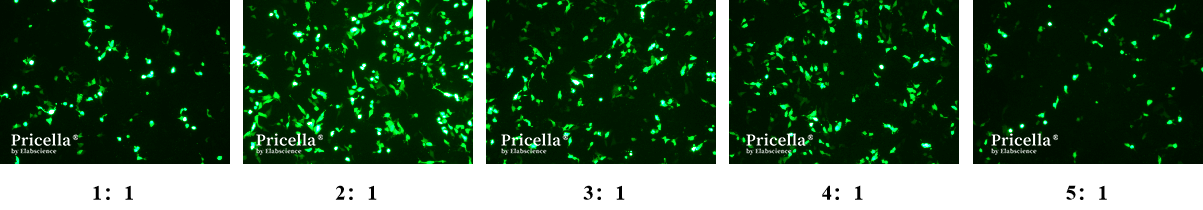
Fig.1 EGFP Expression in Hep G2 Cells with Different Ratios of Transfection Reagent to Plasmid
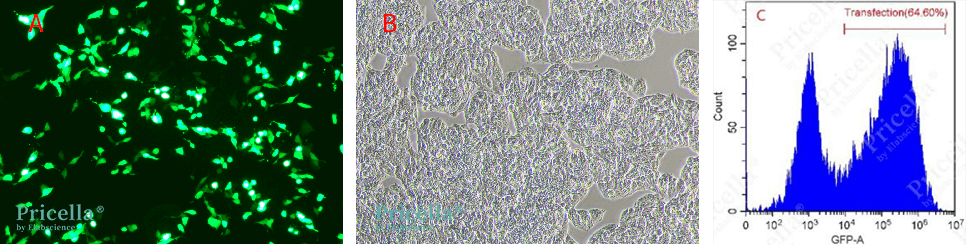
Fig. 2 Fluorochrome protein expression in Hep G2 cells 48 h after transfection with EGFP expression plasmid (A: Brightfield image, B: Fluorescence image, C: Flow cytometry analysis of transfection efficiency)
Precautions
Cell State: The state of the cells is one of the key factors affecting transfection efficiency. Ensure that cells in the logarithmic growth phase with good viability are used, and avoid using cells with too many passages or contamination.
Quality and Purity of Nucleic Acids: The quality and purity of nucleic acids are crucial for transfection outcomes. High-purity nucleic acids that are free from endotoxins should be used.
Experimental Design: When optimizing transfection conditions, it is recommended to set up empty vector controls, untransfected groups, and positive control groups to eliminate confounding variables and ensure the reliability of experimental results.
Cell Type and Transfection Conditions: Different cell types have varying requirements for transfection methods and conditions. Adjustments should be made based on the specific cell type and experimental objective.
References
[1] Fus-Kujawa A, Prus P, Bajdak-Rusinek K, Teper P, Gawron K, Kowalczuk A, Sieron AL. An overview of methods and tools for transfection of eukaryotic cells in vitro. Frontiers in Bioengineering and Biotechnology. 2021 Jul 20; 9: 701031.
[2] Zhi D, Bai Y, Yang J, Cui S, Zhao Y, Chen H, Zhang S. A review on cationic lipids with different linkers for gene delivery. Advances in Colloid and Interface Science. 2018 Mar; 253: 117-140.
[3] Paecharoenchai O, Niyomtham N, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Yingyongnarongkul BE, Opanasopit P. Structure relationship of cationic lipids on gene transfection mediated by cationic liposomes. AAPS PharmSciTech. 2012 Dec; 13 (4): 1302-8.
[4] Huang QD, Ren J, Ou WJ, Fu Y, Cai MQ, Zhang J, Zhu W, Yu XQ. Cationic lipids containing cyclen and ammonium moieties as gene delivery vectors. Chemical Biology & Drug Design. 2012 Jun; 79 (6): 879-87.
[5] O'Brien JA, Lummis SC. Nano-biolistics: a method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles. BMC Biotechnology. 2011 Jun 10; 11: 66.
[6] Gödecke N, Hauser H, Wirth D. Stable expression by lentiviral transduction of cells. Methods in Molecular Biology. 2024; 2810: 147-159.
Prev: Decoding Astrocytes: Essential Techniques for Primary Cell Isolation
Next: Preventing Seasonal Contamination: Step-by-Step Strategies for Stable Cell Culture





