Culturing Bone Marrow-Derived Dendritic Cells (DCs)
Source: PricellaPublished: 2024-12-31
Dendritic cells (DCs) are named for their pseudopod-like or dendrite-like projections. They are the most versatile and specialized antigen-presenting cells (APCs) in the body, with a unique ability to activate naïve T cells and initiate primary immune responses.
DCs are present in extremely low quantities in vivo, which has significantly limited their research and application. However, DCs can be differentiated from precursor cells in various tissues, including bone marrow, peripheral blood, and umbilical cord blood.
In addition to inducing immune responses, DCs also play a role in inducing immune tolerance. Based on their developmental and maturation states, DCs are classified into immature DCs (imDCs), mature DCs (mDCs), and semi-mature DCs (smDCs). DCs express specific surface markers such as OX62 and exhibit high levels of MHC II, CD80, and CD86. Mature DCs are characterized by their strong ability to stimulate allogeneic T cell proliferation.
This article outlines protocols applicable to mouse bone marrow-derived DCs and rat bone marrow-derived DCs.
Extraction Procedure
Isolate the femurs and flush out the bone marrow to obtain a bone marrow cell suspension. Perform density gradient centrifugation to isolate the bone marrow mononuclear cells.
Induction Procedure
Use recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) and recombinant interleukin-4 (IL-4) to induce immature DCs (imDCs). For further maturation, add recombinant tumor necrosis factor-alpha (TNF-α) and continue culturing in vitro to obtain mature DCs (mDCs).
Morphological Characteristics
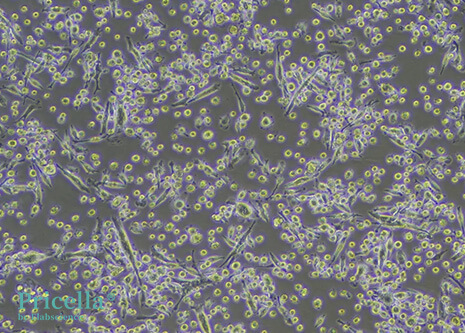
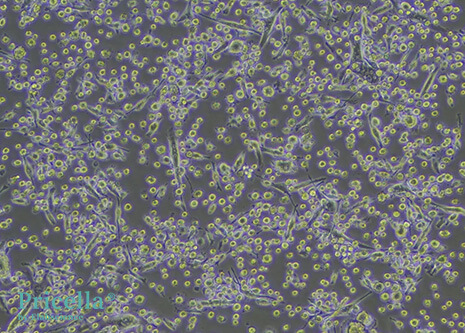
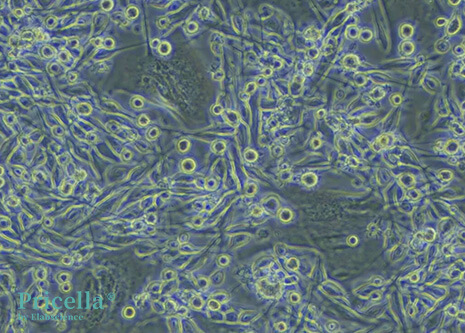
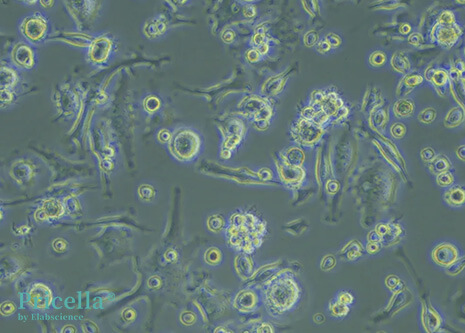
Mature DCs display diverse morphologies. They exhibit irregular shapes with dendrite-like projections of varying length and thickness. Some cells appear leaf-shaped or star-shaped, with a large, translucent appearance.
Figure 1: Mouse Bone Marrow-Derived DCs (100X)
Figure 2: Mouse Bone Marrow-Derived DCs (200X)
Culture Considerations
Culturing DCs requires an extended incubation period. Over time, DCs transition from adherent growth to a semi-suspended and eventually fully suspended state. When DCs begin to grow in suspension, the culture medium removed during media changes may inadvertently contain DCs. To minimize cell loss, collect the spent medium during media changes, centrifuge the medium to recover the cells, and resuspend them in fresh medium for continued culture.
Protocol for Semi-adherent, Semi-suspended Cell Detachment
If detachment and transfer of cells to multi-well plates or other vessels are required before the experiment, follow these steps:
1) Remove the old medium and transfer it to a centrifuge tube. Wash the culture flask twice with PBS, transferring the PBS washes to the same tube. Centrifuge at 1,200–1,500 rpm for 3 minutes, discard the supernatant, and collect the cell pellet (Pellet ①).
2) Add 1 mL of 0.25% trypsin solution to the T25 culture flask. Gently tilt the flask to ensure the trypsin covers the entire surface. Remove the excess trypsin and incubate the flask in a 37°C water bath for 1–3 minutes. Under an inverted microscope, observe the cells retract and round up.
3) Add 5 mL of complete medium to neutralize the trypsin. Gently pipette to dislodge the cells and transfer the cell suspension to a centrifuge tube. Centrifuge at 1,200–1,500 rpm for 3 minutes, discard the supernatant, and collect the cell pellet (Pellet ②).
4) Resuspend Pellet ① and Pellet ② in 5 mL of fresh complete medium, mixing thoroughly.
5) Gently pipette to disperse the cells and seed them into the desired experimental vessels, adding fresh medium as needed. Incubate the cells at 37°C, 5% CO₂, and saturated humidity.
6) Once the cells stabilize, proceed with your experiments. Change the medium every 2–3 days with fresh complete medium.
Prev: Starting from Scratch: Culturing HMC3 Human Microglia Cells