Starting from Scratch: Culturing HMC3 Human Microglia Cells
Source: PricellaPublished: 2024-12-31
Basic Information
• Culture Conditions
Culture Medium: MEM (with NEAA) + 10% FBS + 1% P/S
CO2 Incubator: Stable temperature (37°C), stable CO2 level (5%)
• Growth Characteristics
Adherent, macrophage-like
• Subculturing Ratio and Frequency
Subculture at a 1:3 to 1:5 ratio every 3 to 4 days.
• Cell Type
Microglia
• Morphology
Macrophage-like
Background Information
The HMC3 cell line was established through SV40-dependent immortalization of primary microglia cultures derived from human fetal brain tissue. In resting HMC3 cells, the microglia/macrophage marker IBA1 is strongly positive, while the astrocyte marker GFAP is negative.
Markers of activated microglia, such as MHCII, CD68, and CD11b, are negative in resting HMC3 cells but are upregulated upon activation with IFN-γ (10 ng/mL, 24 hours).
Specific Applications
Neuroscience, Neuroinflammation
Transformed human microglial cells represent a homogenous cell population capable of unlimited growth, providing a convenient system for biochemical analysis of microglial function. As demonstrated in experiments, these cells can also undergo transfection, which is crucial for studying the regulation of gene expression in microglia.
Culture Considerations
1. Change the medium every 2-3 days.
2. Cell debris may form during culture; remove it promptly by changing the medium.
Subculturing Procedure
1. Aspirate the old culture medium.
2. Add approximately 2 mL of PBS, gently swirl the culture flask to rinse the cells, and aspirate the PBS to discard.
3. Add about 1 mL of trypsin, gently swirl the flask to ensure all cells are covered.
4. Place the flask in the incubator for 2-3 minutes to allow dissociation. Gently tap the side of the flask. Once the cells detach and the cell clusters start to disperse under the microscope, stop the dissociation.
5. Add 3 mL of serum-containing medium to stop the dissociation. Pipette the cells to detach them completely, and pipette repeatedly to create a homogeneous cell suspension. You can observe this under the microscope.
6. Collect the cell suspension and centrifuge at 1200 rpm for 3 minutes. After centrifugation, aspirate and discard the supernatant.
7. Add fresh culture medium and pipette to mix the cells. Seed the cells into a new culture flask as needed, add sufficient medium, and loosely cap the flask or use a vented cap for culture.
8. Check the incubator for CO2 levels, temperature, and water tray.
Medium Change Procedure
1. Aspirate the old medium, rinse with PBS, and then add fresh medium.
2. Change the medium every 2-3 days.
Cryopreservation Procedure
1. Prepare the freezing medium, the recommended composition is:
55% basal medium (DMEM/F12) + 40% FBS +5% DMSO
2. DMSO generates heat when mixed, so be sure to let the freezing medium cool down before use to prevent cell damage.
3. Dissociate the cells to obtain a cell suspension.
4. Centrifuge the cell suspension at 1200 rpm for 3 minutes. Carefully remove and discard the supernatant, ensuring it is as dry as possible.
5. Resuspend the cell pellet in the prepared freezing medium. For a fully confluent T25 flask, it is recommended to freeze one vial, or count the cells and freeze at 3-5 × 106 cells per vial.
6. Transfer the aliquoted cells into a programmed freezing container and place it in a -80°C freezer overnight.
7. The next day, retrieve the vials from the -80°C freezer and quickly transfer them to liquid nitrogen for long-term storage.
Thawing Procedure
1. Preheat a water bath to 37°C. Prepare clean disposable PE gloves and add 5 mL of sterile culture medium to a sterile centrifuge tube.
2. Remove the cells from the liquid nitrogen tank and place them in PE gloves. Quickly immerse the vial in the water bath, gently shaking to thaw the cells rapidly—ideally within 1 minute.
3. In a biosafety cabinet, transfer the thawed cell suspension into the centrifuge tube containing fresh medium. Centrifuge at 1200 rpm for 3 minutes, then carefully remove and discard the supernatant.
4. Resuspend the cell pellet in the appropriate complete culture medium and transfer it into a sterile container (such as a culture flask or dish). Add the culture medium as needed and transfer to an incubator for cultivation .
5. Thaw the cells quickly to minimize the time they are exposed to room temperature. Promptly centrifuge to remove DMSO from the solution.
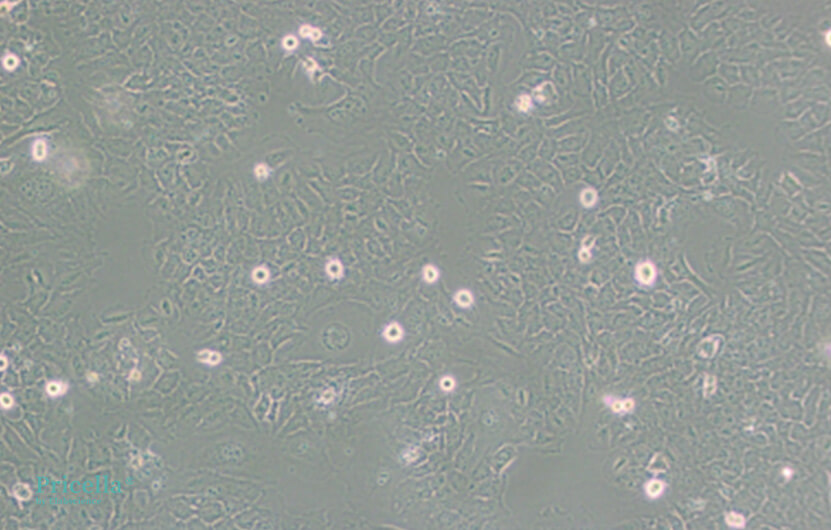

Figure 1. Normal Growth of HMC3 Cells
Prev: Morphology, Growth, and Troubleshooting in BV2 Cell Culture





