How to Optimize the Culturing of H9c2 (2-1) Cells
Source: PricellaPublished: 2025-01-02
H9c2 (2-1) cells, derived from embryonic BD1X rat heart tissue, are a subclone of the original rat cardiomyocyte cell line and exhibit many characteristics of skeletal muscle. The myoblasts in this cell line can fuse to form multinucleated myotubes and respond to acetylcholine stimulation. If the serum concentration in the culture medium drops to 1%, H9c2 (2-1) cells will fuse rapidly. This cell line is an effective model system for studying cellular development[1].
In this issue, we summarize key points to consider when culturing H9c2 (2-1) cells.
Cell Thawing
The thawing process should be swift; thawed cryopreserved cells should not be left at room temperature for too long and need to be centrifuged promptly to remove DMSO.
Cell Cryopreservation
Recommended freezing medium: 55% basal medium + 40% FBS + 5% DMSO.
When cryopreserving cells, try to disperse them evenly and be mindful of the pipetting force.
Cell Culturing
H9c2 (2-1) cells are adherent cells with a normal spindle shape.
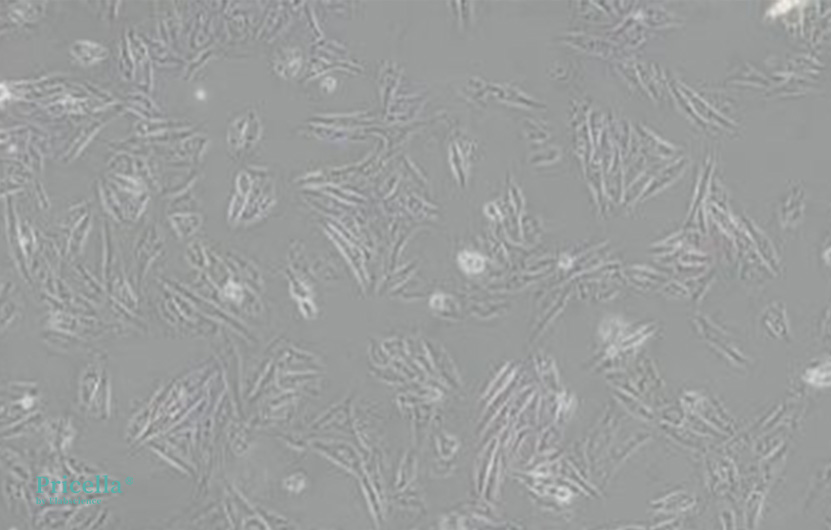
Figure 1. Normal morphology of H9c2 (2-1) cells
Culture Conditions
❖ Culture Medium: DMEM + 10% FBS + 1% P/S
❖ Recommended Passage Ratio: 1:2 to 1:4
❖ Recommended Medium Change Frequency: 2-3 times per week
❖ Incubator Conditions: 37°C, 5% CO2
Passaging Procedure
❖ Remove the culture medium from the dish and rinse with PBS, then discard the supernatant.
❖ Add 0.25% trypsin to rinse, then discard the supernatant.
❖ Incubate in a CO2 incubator for 2-4 minutes for dissociation.
❖ Add complete growth medium to stop the dissociation, and gently pipette to ensure even cell distribution.Common Issues and Solutions
1. Why are there many granules on the cell surface?
The culture environment may be problematic. Switching to DMEM/F-12 medium might help, and after three passages, the cell surface should smooth out.
2. Why do vacuoles appear?
This could be due to uneven cell seeding, causing localized overcrowding, which deteriorates cell condition and increases vacuoles. It might also be due to poor-quality serum, so consider switching to high-quality serum and changing the medium promptly.
3. Why is cell growth slow?
This could be due to low seeding density. Consider re-culturing and then re-seeding at a higher density.
4. Why are the cells in poor condition and easily detached?
This could be a serum issue. Consider replacing the serum and ensure it is thawed properly using a gradual process, not a water bath. Overcrowding could also be a factor, so passage the cells when some areas are becoming too dense, rather than waiting for full confluence.
Important Notes
❖ Maintain a confluency range of 30% to 90% during culture.
❖ Handle all steps gently to avoid damaging the cells.
❖ Use high-quality culture media to minimize harmful effects on the cells.
Reference
[1] Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Experimental Cell Research. 1976 Mar 15;98(2):367-81.Prev: Culturing Bone Marrow-Derived Dendritic Cells (DCs)
Next: Classification and Selection Criteria for Cytokine Products





