Comprehensive Guide to Neural Stem Cell Cultivation and Differentiation
Source: PricellaPublished: 2025-02-27
Neural stem cells (NSCs) play a pivotal role in neuroscience research due to their unique proliferative capacity and potential for multilineage differentiation. However, cultivating NSCs presents various challenges, such as stringent culture requirements and difficulty in controlling differentiation. So, how do you choose the right cultivation method? What are the impacts of different culture conditions on cell states? In this article, we’ll provide an in-depth look at neural stem cell cultivation methods, differentiation strategies, and address common experimental challenges to help you advance your research.
I. Introduction to Neural Stem Cells
Neural stem cells can be isolated from various tissues, including embryos, the cerebral cortex, the hippocampus, and the spinal cord. To successfully culture NSCs in vitro, an appropriate culture system is crucial. Factors such as culture medium composition, use of supplements, and culture conditions profoundly influence the proliferation, survival, and differentiation of NSCs.
• Growth Medium
Common formulation: DMEM/F12 + B-27 (without Vitamin A) + bFGF + EGF + P/S
To better meet the cultivation needs of NSCs, Pricella® offers a range of optimized neural stem cell complete culture media, providing a more efficient and stable cultivation experience.
• Culture Conditions
Atmosphere: 95% Air, 5% CO2
Temperature: 37°C
II. Neural Stem Cell Cultivation Methods
There are two main methods for cultivating neural stem cells: suspension culture and adherent culture. Each has its own advantages and is suited to different research needs.
• Suspension Culture
NSCs are cultured in non-TC-treated, uncoated, or low-adhesion vessels with serum-free medium, naturally forming neural spheres.
Disadvantages | |
Forms stable neural spheres, maintains undifferentiated state, favorable for long-term culture and large-scale expansion | Large cell spheres can form, leading to hypoxia and nutrient depletion at the center, causing cell death |
Faster proliferation rate in early stages compared to adherent culture | Autophagy levels increase, possibly due to nutrient shortages within neural spheres |
Simple culture conditions and high success rate of cell isolation | Frequent centrifugation and resuspension increase cell loss |
Stronger stemness maintenance, less differentiation compared to adherent culture | Not ideal for differentiation induction and observing differentiated morphologies |
• Adherent Culture
After digesting and dissociating the neural stem cell spheres into single cells, they are seeded into TC-treated and coated vessels and cultured in serum-free medium to form a monolayer of cells.
Advantages | Disadvantages |
Forms well-defined monolayers, lower passage loss, easier to observe cell morphology and differentiation stages, better for NSC cultivation and storage | Susceptible to spontaneous differentiation during long-term culture |
NSCs in monolayer form, with more surface area in contact with the medium, higher success rate in differentiation induction | |
NSCs retain strong proliferative capacity and multilineage differentiation potential even after multiple passages | NSCs may detach from surface easily, requiring coated vessels; passaging is complex and may cause cells to form spheres again after passaging |
Lower autophagy levels, which helps maintain cell stability and proliferative ability |
III. Neural Stem Cell Differentiation Induction
1. Induction of Astrocytes
After dissociating the neural stem cell spheres, resuspend the cells in astrocyte induction medium (DMEM High Glucose + B-27, with Vitamin A + N-2 + GlutaMAX + FBS + bFGF) and seed into poly-L-lysine-coated culture dishes. Over time, the NSCs will differentiate into astrocytes, as shown in Figure 1.
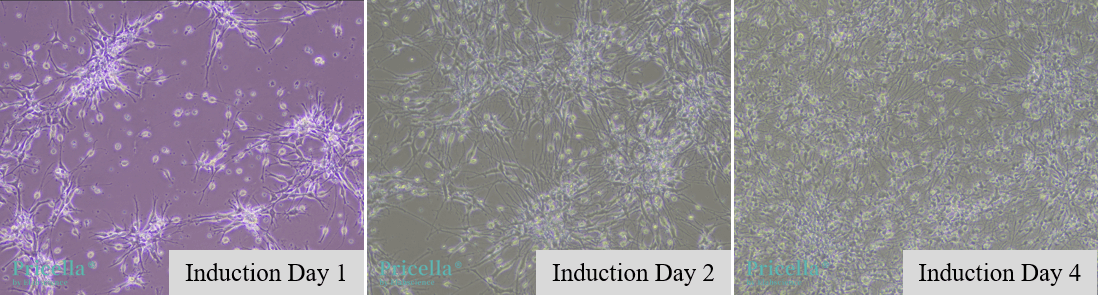
Figure 1. Morphological changes during the induction of neural stem cells into astrocytes.
2. Induction of Oligodendrocytes
Similar to astrocyte induction, resuspend the NSCs in oligodendrocyte induction medium (DMEM/F12 + B-27, with Vitamin A+ EGF + bFGF + T3 + Insulin) to induce differentiation into oligodendrocytes, as shown in Figure 2.
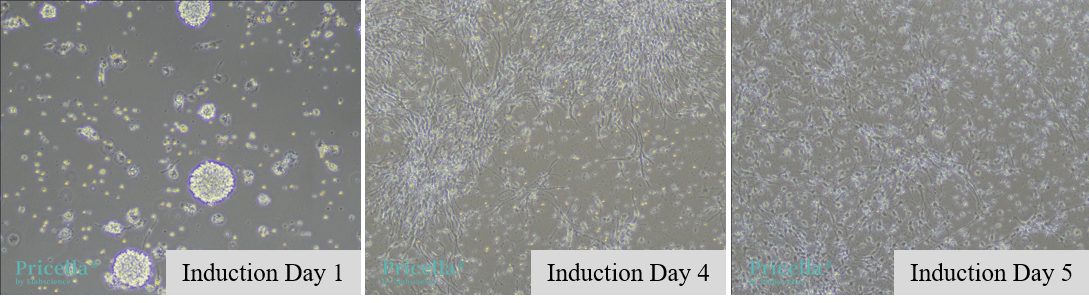
Figure 2. Morphological changes during the induction of neural stem cells into oligodendrocytes.
3. Induction of Neurons
After dissociating the neural stem cell spheres, resuspend the cells in neuron induction medium (Neurobasal + 1% N-2 + 2% B-27, with Vitamin A + NEAA + β-mercaptoethanol + L-glutamine). After 6 days, add 10 ng/mL GDNF and 10 ng/mL BDNF to further promote neuronal differentiation.
IV. Common Issues in Neural Stem Cell Cultivation
1. How do you accurately determine the right time to passage neural stem cells?
The timing for passaging should be based on the size and morphology of the neural stem cell spheres. The center of the spheres should remain bright, and cells should not grow too large. For example, Figure 3 shows an overgrown sphere that should be passaged before it darkens in the center. Generally, after a 1:2 passage, passaging can be done every 2-3 days.
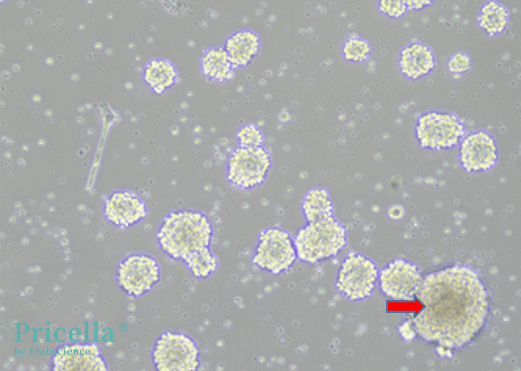
Figure 3. Neural stem cell sphere growth morphology.
2. Is complete digestion to single cells necessary for neural stem cells that are difficult to dissociate?
For suspension culture, complete dissociation to single cells is not required. However, for adherent culture, dissociation into single cells is necessary to promote adhesion. It is recommended to use Accutase enzyme instead of trypsin, as it is milder and reduces the risk of cell damage.
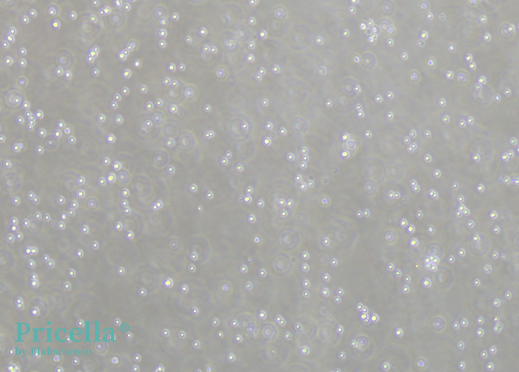
Figure 4. Single-cell dissociation using Accutase enzyme.
3. How should medium changes be performed in suspension culture?
It is recommended to use a half-volume medium change. The specific steps are: place the culture vessel vertically, allow most cells to settle, then aspirate half of the spent medium and replace with fresh medium. To reduce cell loss, you can transfer the spent medium to a centrifuge tube, centrifuge at 500 rpm for 5 minutes, discard the supernatant, resuspend the cells in fresh medium, and return to the original vessel.
Whether using suspension or adherent culture, each method has its advantages and drawbacks. Researchers can choose the most suitable approach depending on their experimental needs. Additionally, mastering the correct differentiation induction strategies and optimizing culture conditions can significantly enhance the success rate of neural stem cell cultivation.
Prev: Troubleshooting CTLL-2 Cell Culture Challenges
Next: Cell STR Authentication: The Gold Standard for Ensuring Cell Identity





