Cell STR Authentication: The Gold Standard for Ensuring Cell Identity
Source: PricellaPublished: 2025-03-04
In the fields of life sciences and biomedical research, the accuracy of cell identity directly impacts the reliability of experimental results. Short Tandem Repeat (STR) profiling has become the widely accepted "gold standard" for cell authentication, offering high precision in verifying cell identity. STR analysis is essential not only for initial authentication when cells are first banked but also for continuous monitoring during long-term cell culture. But how often should STR authentication be performed during cell culture? How can we ensure the accuracy and validity of STR results? In this issue of Cell Culture Academy, we will provide an in-depth look into the necessity and scientific foundation of STR authentication, helping you maintain confidence in your cell cultures.
1 Defining the Purpose of STR Authentication
The first step in STR authentication is to clearly define the objective and requirements, which generally fall into the following categories:
1.1 Initial Testing for New Cells Entering the Cell Bank
If the identity of the cells needs to be confirmed, it is recommended to use STR authentication to perform a blind match against an existing database. The match results can help confirm the true identity of the cells. When selecting a database, ensure it includes all STR loci information for the target cells; otherwise, the match may be inaccurate or incorrect.
1.2 Routine Authentication during Cell Culture
For cells that have been cultured for a long time, regular STR authentication helps monitor the stability of cell identity. If there is an earlier STR report, comparing the current report with it can help detect any changes in loci information. For example, if new peaks appear in the current report, this could indicate chromosome mutations or minor contamination by other cells during culture. If contamination is suspected, it is recommended to re-perform STR testing after 3-5 passages to further confirm the results.
1.3 Authentication of Primary Cells or Newly Established Cell Lines
For primary cells or self-established cell lines, STR authentication ensures that the cells have not been contaminated by other known cell lines during the establishment process. STR authentication also helps establish a baseline reference for future use [1].
2 Sensitivity and Limitations of STR Authentication
Laboratory data indicate that STR profiling can detect intra-species cross-contamination at a sensitivity threshold of approximately 10%. In other words, if contamination levels are below 10%, significant peak alterations may not appear in the STR results (as shown in Figure 1). Even if minor changes occur, low-intensity peaks may be automatically filtered out by analysis software. Therefore, when cross-contamination is detected via STR profiling, it often means that the contamination level is already relatively high. However, a single STR test only reveals the current contamination status and cannot predict future risks.
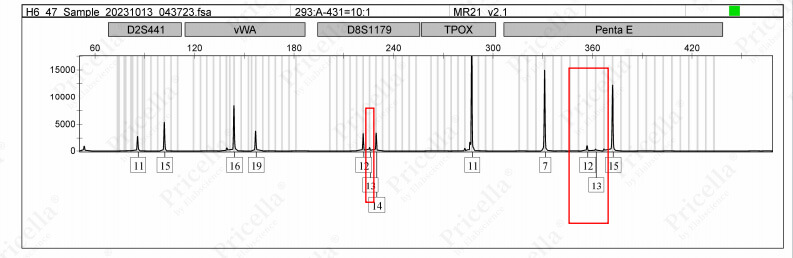
Figure 1: Cross-contamination pattern for human cells with 10% contamination
Recommended Solution:
To mitigate the risk of undetected low-level contamination, we recommend performing STR authentication every 3–6 months for cell banks. If cross-contamination occurs during subculturing, the proportion of contaminating cells will not remain constant; rather, cells with a growth advantage will gradually outcompete others. This selection process is closely related to cell proliferation rates. Typically, after 5–10 passages, differences between cell populations become more pronounced, allowing STR profiling to effectively detect changes and ensure timely intervention.
3 STR Authentication Standards for Mouse Cell Lines
For mouse cell lines, there are two STR profiling methods based on the number of loci analyzed:
l 9-locus method: Includes loci 4-2, 5-5, 6-4, 6-7, 9-2, 12-1, 15-3, 18-3, and X-1.
l 18-locus method: Includes loci 1-1, 1-2, 2-1, 3-2, 4-2, 5-5, 6-4, 6-7, 7-1, 8-1, 11-2, 12-1, 13-1, 15-3, 17-2, 18-3, 19-2, and X-1.
In 2023, the NIH published Authentication of Human and Mouse Cell Lines by Short Tandem Repeat (STR) DNA Genotype Analysis in the Assay Guidance Manual [2], officially recommending the 18-locus STR method for mouse cell line authentication.
In human cell line authentication, a match rate of ≥80% is generally considered indicative of identity due to the genetic diversity among human populations. Even closely related individuals exhibit significant STR profile variations. However, laboratory mice used in research are often inbred strains, isogenic strains, or genetically related strains, resulting in less genetic diversity. Consequently, STR match rates among different individual-derived mouse cell lines may exceed 80%.
In such cases, STR results should be supplemented with additional validation methods, including cell background information, growth characteristics, and phenotypic traits, to confirm cell identity.
STR Authentication Case Study
The RM-1 and Py230 cell lines originate from genetically related C57BL/6 mice, leading to high similarity in their STR profiles. Furthermore, the RM-1 cell line database includes only 8 STR loci, which can lead to an artificially higher match rate when compared to Py230 cells.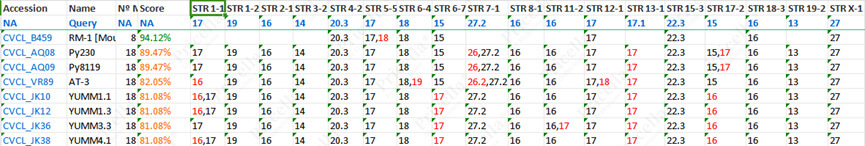
Figure 2: STR authentication data match table for Py230 cells
Similarly, RM-1 and MOC1 cells originate from the same C57BL/6 mouse strain, resulting in over 80% similarity in their STR profiles.
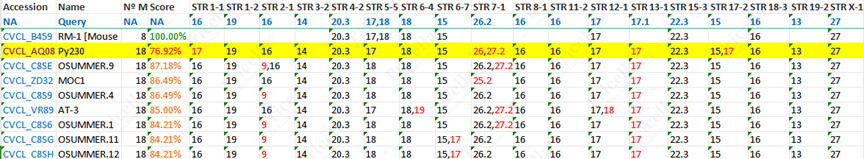
Figure 3: STR authentication data match table for RM-1 cells
STR authentication plays a crucial role in confirming cell identity, detecting cross-contamination, and verifying cell line stability. To ensure experimental reliability and reproducibility, STR profiling should be performed not only at the time of initial cell acquisition but also periodically during long-term culture and passaging. By implementing rigorous STR authentication practices, researchers can maintain high cell line purity and stability, laying a solid foundation for high-quality scientific research.
References
[1] ICLAC, Guide to Human Cell Line Authentication.
[2] Almeida JL, Korch CT. Authentication of Human and Mouse Cell Lines by Short Tandem Repeat (STR) DNA Genotype Analysis. In: Markossian S et al. (editors). Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2023. Available at: https://www.ncbi.nlm.nih.gov/books/NBK144066/Prev: Comprehensive Guide to Neural Stem Cell Cultivation and Differentiation
Next: Cell Species Identification: Safeguarding the Pure Lineage of Research Cells





