Choosing the Right Transfection Method: Transient Transfection vs. Stable Transfection
Source: PricellaPublished: 2025-03-13
Transfection is a critical technique in life sciences that allows cells to actively or passively take up exogenous genetic material (DNA or RNA) under specific conditions, enabling them to acquire new phenotypic traits. Conventional transfection techniques can be categorized into two main types: transient transfection and stable transfection.
In this article, we will provide a comprehensive comparison of the principles, procedures, and methods of these two transfection approaches to help you choose the most suitable technique for your experiments.
Comparing the Principles of Transient and Stable Transfection
Transient Transfection
Transient transfection involves introducing exogenous genetic material (DNA or RNA) into cells, but without the integration of this material into the host genome. As cells grow and divide, the exogenous genetic material is gradually diluted and eventually lost, resulting in the loss of gene function. This method is typically used for experiments requiring rapid, short-term expression of the transgene.
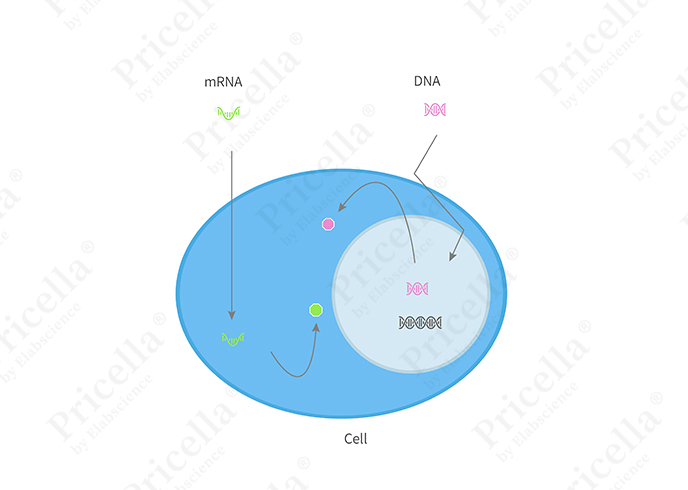
Figure 1. Illustration of transient transfection
Stable Transfection
Stable transfection refers to the integration of foreign DNA into the host cell’s chromosomes using specific techniques. This ensures that the target gene can be stably expressed over the long term. In stable cell lines, the foreign gene is consistently expressed in subsequent generations of cells. However, the integration process is often random and influenced by the complex regulatory mechanisms of the cell, leading to relatively low transfection efficiency. By employing carefully designed selection strategies, cell clones that stably express the foreign gene can be obtained. Choosing the right transfection strategy and selection method is crucial for success.
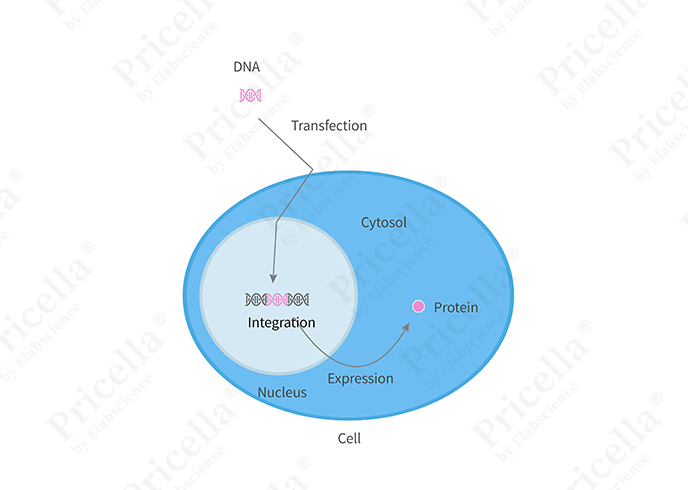
Figure 2. Illustration of stable transfection
Procedural Comparisons: Transient vs. Stable Transfection
Transient and stable transfection share several similar steps but differ significantly in experimental timelines and procedural complexity. Below is an overview of the workflow for each method (Figures 3 and 4).
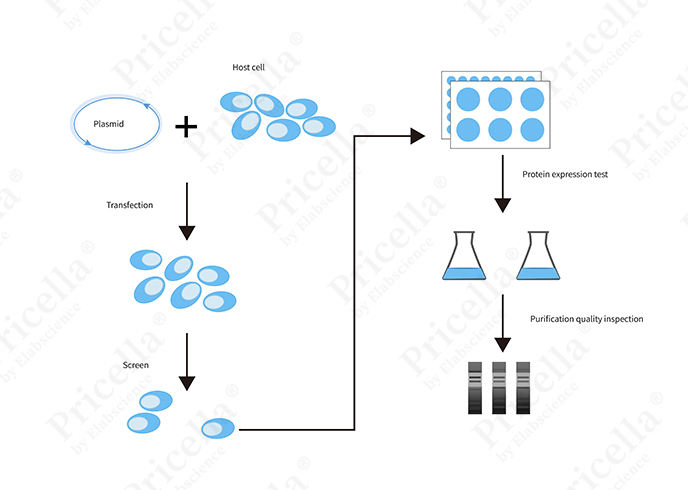
Figure 3. Workflow diagram for transient transfection
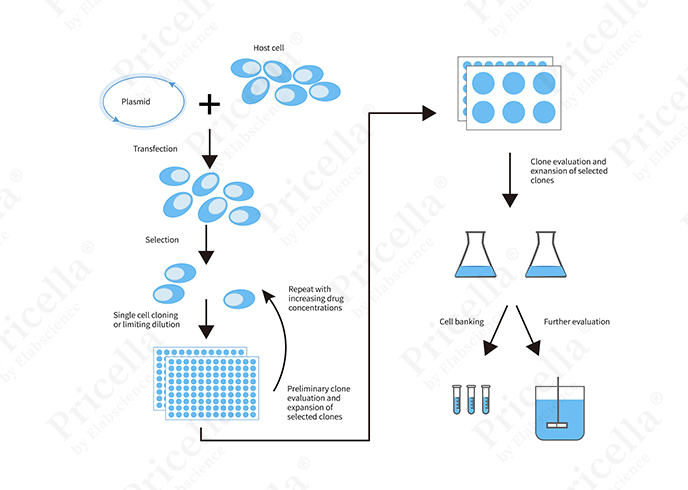
Figure 4. Workflow diagram for stable transfection
Similarities
01 Cell Preparation: Both methods require selecting an appropriate cell line and culturing it to the logarithmic growth phase to ensure optimal conditions for transfection.
02 Plasmid Selection: Plasmid design should match the experimental requirements, considering factors such as plasmid size, functional elements, origin of replication, and compatibility with the cell type. The plasmid concentration and purity should meet transfection srequirements.
03Choice of Transfection Method: Depending on the experimental goals and cell type, different transfection techniques can be chosen, such as physical methods (e.g., electroporation), chemical methods (e.g., liposome-mediated transfection), or biological methods (e.g., lentiviral infection).
04 Transfection Procedure: The transfection procedure varies depending on the method used. For example, chemical or biological transfection methods typically involve mixing the plasmid DNA with the transfection reagent to form a complex, which is then added to the cell culture. In physical methods like electroporation, plasmid DNA is suspended in a specific buffer and introduced into cells using an electric field.
Differences
Stable transfection involves additional steps not required in transient transfection:
01 Antibiotic Selection: Antibiotic resistance markers are used to select cells that have successfully undergone stable transfection.
02 Clone Selection: Single-cell clones are isolated and expanded to ensure stable gene expression.
03 Cell Expansion and Validation: Multiple rounds of selection and experimental validation are performed to confirm stable gene expression.
04 Long-term Maintenance and Monitoring: Cells are periodically monitored to ensure consistent gene expression and cellular functionality over time.Transfection Methods: Chemical, Physical, and Biological Approaches
The cell membrane is composed of a negatively charged phospholipid bilayer, which acts as a barrier to macromolecules, especially negatively charged ones like DNA and RNA. Researchers have developed various transfection techniques to help these macromolecules penetrate this barrier, which can be broadly categorized into chemical, physical, and biological methods. The choice of transfection technique significantly impacts the outcome, and the appropriate method varies between transient and stable transfection.
• Chemical Transfection
This method uses positively charged carrier molecules to form complexes with negatively charged nucleic acids, allowing cellular uptake via endocytosis.
Transfection Technique | Key Features | Applications |
Liposome-mediated Transfection | Widely applicable; high reproducibility; cell-specific effects; efficiency influenced by cell type | Transient/Stable Transfection |
Calcium Phosphate Method | Simple operation; low reproducibility; unsuitable for primary cells | Transient/Stable Transfection |
DEAE-gextran Method | Moderate toxicity; generally used for specific cell types; unsuitable for primary cells | Transient Transfection |
Cationic Polymers Method (e.g., PEI, PLL) | Suitable for both adherent and suspension cells; efficiency affected by serum and additives | Transient/Stable Transfection |
Physical Transfection
This method creates temporary pores in the cell membrane to facilitate the entry of genetic material.
Transfection Technique | Key Features | Applications |
Electroporation Method | Broad applicability; high cell death rate; requires large amounts of DNA | Transient/Stable Transfection |
Microinjection Method | High technical difficulty; limited number of transfected cells; narrow application range | Transient/Stable Transfection |
Biological Transfection
This method utilizes genetically engineered viruses to introduce non-viral genes into cells.
Transfection Technique | Key Features | Applications |
Adenovirus | Suitable for hard-to-transfect cells; requires host specificity; safety concerns | Transient Transfection |
Retrovirus | Suitable for hard-to-transfect cells; limited gene capacity; host specificity; safety concerns | Stable Transfection |
Lentivirus | Long process; suitable for specific host cells; widely used in gene editing | Transient/Stable Transfection |
Choosing Between Transient and Stable Transfection
As discussed, transient and stable transfection have distinct principles, procedures, and applicable transfection techniques. The choice between the two methods should consider factors such as experimental requirements, cost, and time constraints.
Transient transfection is typically used for experiments requiring rapid results, such as observing the effects of gene overexpression or silencing. Cells are usually collected within 24-96 hours post-transfection. This method is ideal for short-term, low-cost experiments where fast results are needed.
Stable transfection, on the other hand, requires a longer timeframe, typically 2-3 weeks or more. It is better suited for long-term studies such as pharmacological research, gene therapy, or experiments involving gene editing (e.g., insertion or knockout).In summary, transient transfection is characterized by simplicity, shorter timelines, and lower costs, making it ideal for short-term experiments. In contrast, stable transfection, while more complex, time-intensive, and costly, is indispensable for long-term studies, gene therapy applications, and in-depth exploration of gene function.
Prev: Cell Scratch Assay: Unveiling the Secrets of Cell Migration!
Next: Cell Culture Academy| Reasons and Solutions for Adherent Cell Detachment





