Cell Culture Academy| Reasons and Solutions for Adherent Cell Detachment
Source: PricellaPublished: 2025-03-14
Have you ever encountered a situation where a large number of adherent cells detachment during the cell culture process? Often, seemingly minor details are overlooked, yet these details are the key factors causing cell detachment. So, how can we effectively prevent and address this issue? In this edition of Cell Culture Academy, we have compiled several common factors that affect cell adhesion and have provided corresponding solutions to help you optimize culture conditions, ensuring your cells adhere and grow smoothly!
1. Improper Digestion During Subculturing
Inadequate or excessive trypsin digestion can negatively impact cell adhesion. For cells that are easily digested, it is recommended to use 0.25% trypsin with EDTA, which typically requires 2-3 minutes for complete digestion. For cells that are more difficult to digest (such as A-431 and MCF 10A), the digestion time usually needs to be extended to 5-15 minutes. Before stopping digestion, observe the cell condition under a microscope. Digestion should be halted when the cells become rounded and some cells detach from the side of the flask. Additionally, avoid forcefully tapping or pipetting the flask, as this may damage the cells and affect their adhesion. Figure 1 shows the A549 cells digestion process.
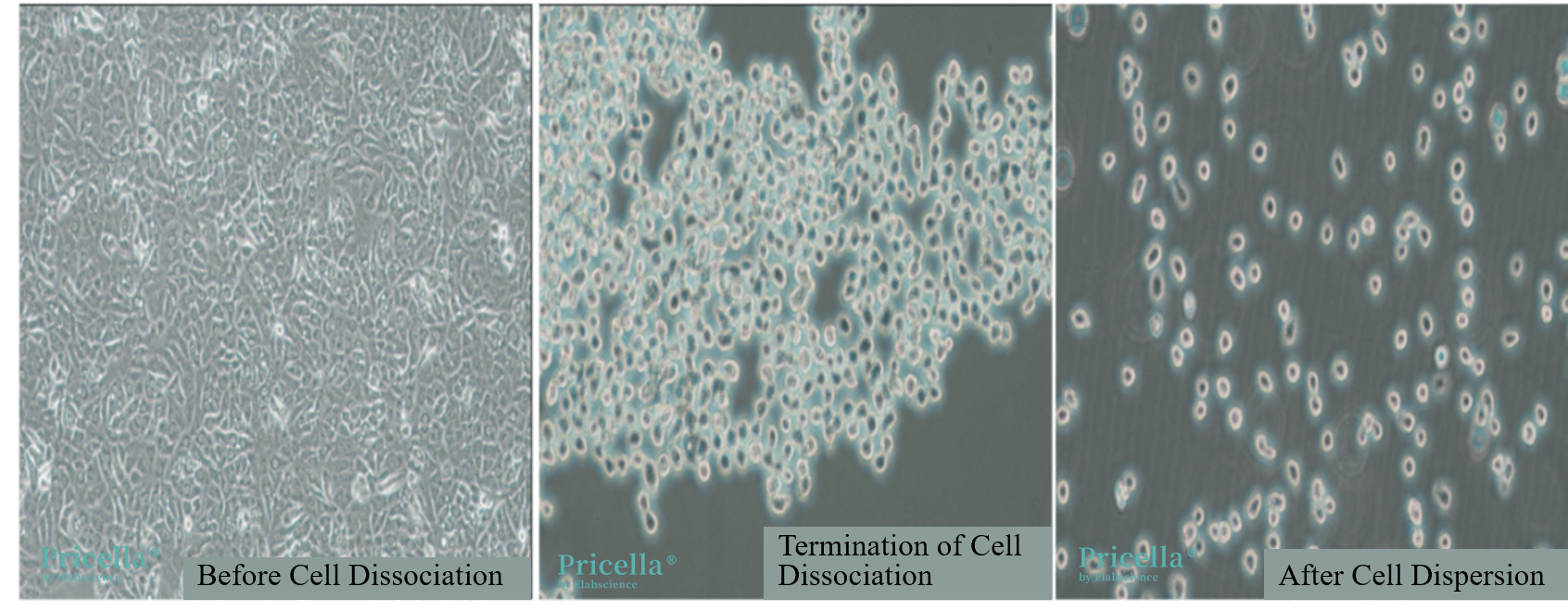
Figure 1. Digestion Process of A549 Cells
2. Cell Growth Density Too High or Too Low
Both excessively high and excessively low cell growth densities can negatively impact cell adhesion. When cell density is too high, competition for space between cells intensifies, leading to rapid nutrient depletion and accumulation of metabolic byproducts, which can compromise cell adhesion. Conversely, when cell density is too low, cell-to-cell interactions and signaling are reduced, slowing down cell growth and potentially causing cells to detach and float. Therefore, it is essential to regularly monitor and adjust cell density during the culture process to ensure that cells grow within an optimal range.
3. Issues related to culture medium
3.1 Significant Fluctuations in Medium pH
Most cell lines grow optimally at a pH of around 7.2-7.4. Conventional culture media contain phenol red as an indicator, allowing for the monitoring of pH changes through variations in the color of the medium. In most cases, fresh medium appears red (as shown in Figure 2, pH 7.4). As cells grow and metabolize, they produce acidic substances, causing the pH of the medium to drop, at which point the medium turns yellow. If the medium appears purple (as shown in Figure 2, pH 7.8), it indicates an increase in pH, and it is necessary to check whether the CO₂ concentration in the incubator and the pressure gauge on the CO₂ tank are normal. If the CO₂ concentration is not the issue, it is also important to consider whether the culture flask has poor gas permeability. When there are significant fluctuations in the pH of the medium, the cause should be promptly investigated, and a medium change should be performed if necessary.
Note: Different types of media may vary in color; for example, DMEM is typically slightly darker than RPMI-1640. When culturing cells, the color change of the medium can be used as a preliminary indicator of cell status.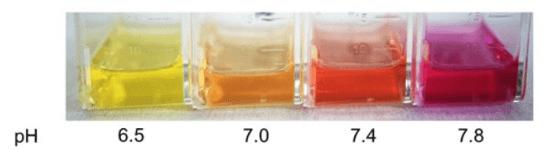
Figure 2. Colors Corresponding to Different pH Levels of Medium (Image sourced from the internet)
3.2 Inappropriate Medium Formulation or Insufficient Nutrients
When purchasing a cell line, the cell bank or manufacturer typically recommends the most suitable medium formulation for optimal cell growth. Altering the components of the medium may lead to suboptimal cell growth, manifesting as slowed proliferation or cell detachment. Additionally, if the complete medium lacks sufficient nutrients, or if the quality of the medium or serum is poor, it may also result in cell detachment. During cell culture, the medium is usually replaced every 2-3 days. If the cell density is high and the medium turns yellow quickly, the frequency of medium changes can be increased appropriately, or the cells should be passaged in a timely manner. Furthermore, expired medium should be avoided to prevent adverse effects on cell attachment and normal growth.
3.3 Changing Serum Brands or Batches
When switching serum brands or batches, cells may experience adaptation issues leading to cell detachment. Avoid frequent changes of serum brands/batches. If replacement is necessary, first conduct a trial test with sample-size serum. If effective, purchase a 6-month or 1-year supply to minimize batch-to-batch variations.
3.4 Insufficient Culture Medium Volume
During cell culture, if the volume of culture medium is too low, excessive evaporation over time can lead to poor cell conditions or even cell death, resulting in cell detachment. The appropriate amount of culture medium should be added based on the size of the culture vessel to ensure an adequate volume throughout the culture process. For routine culture, add 5-6 mL of medium to a 6 cm dish and 10-15 mL of medium to a 10 cm dish.
4. Temperature Fluctuations
Mammalian cells require cultivation at 37℃. Pre-warm media and PBS to 37℃ before medium changes or passaging. Minimize exposure to room temperature during handling to prevent cold-induced cell contraction and detachment.
5. Improper Culture Vessel Handling or Selection
5.1 Lack of Cell Adhesion Promoters
Adherent cells typically attach well under normal conditions. However, a small number of adherent cells (such as ASPC-1, NCI-H2170, etc.) may exhibit floating cells after a medium change due to their inherent characteristics. This phenomenon usually does not require special treatment. On the other hand, for culturing cells with weaker adhesion abilities (such as LNCaP clone FGC, VCaP, U-87 MG, etc.), coating the culture vessels can promote cell attachment and prevent cell detachment. Common coating substrates include poly-L-lysine (D-type and L-type), gelatin, collagen, and extracellular matrix mixtures, which can be selected based on the specific needs of the culture.
5.2 Incorrect Culture Vessel Selection
It is recommended to use TC (Tissue Culture-Treated) vessels for culturing adherent cells, as their surfaces are specially modified to be hydrophilic, which promotes better cell attachment and growth. Non-TC vessels or Ultra-low Attachment surfaces are not suitable for culturing adherent cells.
6. Prolonged Cell Culture Period
Prolonged culture period may gradually deteriorate, with insufficient secretion of adhesion factors, leading to weakened attachment and cell detachment. Therefore, it is important to schedule the culture period according to the cell growth characteristics, avoid cellular aging, and passage the cells in a timely manner to maintain their vitality.
7. Cell Contamination
Cell contamination is a common occurrence during routine culture. Contaminants compete with cells for nutrients, leading to poor cell condition, detachment, and even cell death. Once signs of contamination are detected, a thorough investigation of reagents and the culture environment should be conducted, and effective measures should be taken to address the issue. For less detectable types of contamination, such as mycoplasma or "nanobacteria" contamination, regular testing should be performed, and Anti-Mycoplasma Treatment Reagent or Anti-Nanobacteria Treatment Reagent should be used if necessary.Prev: Choosing the Right Transfection Method: Transient Transfection vs. Stable Transfection
Next: L-929 Cell Culture Guide





