Cell Scratch Assay: Unveiling the Secrets of Cell Migration!
Source: PricellaPublished: 2025-03-04
The cell scratch assay is a simple and cost-effective in vitro technique primarily used to assess the horizontal migration ability of cells in a two-dimensional space. The basic principle is to create a scratch in a monolayer of cells and observe how the cells migrate to repair the scratch, thereby evaluating their migratory ability. This process is closely related to physiological events such as organ formation during embryonic development, wound healing, and immune responses. It is especially critical in cancer research because the migration of cancer cells is a key step in tumor metastasis, which is one of the leading causes of death in solid tumor patients. [1] Therefore, studying cell migration behavior and assessing migratory capacity is of great significance.
In this issue of Cell Culture Academy, we will analyze the advantages and limitations of the cell scratch assay, as well as explore the basic steps and key considerations to help you successfully conduct the experiment.
1. Advantages and Limitations of the Cell Scratch Assay
Advantages
• Simulates the in vivo process of cell migration
• Preserves extracellular matrix and cell-cell junctions
• Can be combined with live-cell imaging to monitor migration in real-time
• Simple to perform, low-cost, and suitable for most laboratories
Limitations
• Compared to other common cell migration assays, the cell scratch assay requires more time for cells to form a monolayer and for wound healing to occur
• Typically uses culture dishes, 6-well or 12-well plates, leading to higher consumption of cells and culture medium, making it less suitable for experiments using hard-to-obtain primary cells or expensive chemicals
• Cannot simulate chemical gradients, and thus cannot replace other methods like the Boyden chamber assay that test for chemotactic migration
2. Basic Steps of the Cell Scratch Assay
1) Cell Culture
Grow cells into a tight monolayer (confluence 90%-100%), ensuring there are no visible gaps between cells.
2) Create the Scratch
Use the tip of a pipette to gently scrape a straight line across the cell monolayer, creating an artificial wound. Then, wash away any detached cells with culture medium or PBS, ensuring the edges of the scratch are even.
Tip: To ensure consistent imaging at different time points, draw a fine line at the bottom of the culture dish or plate (as shown in Figure 1) or lightly etch the bottom of the container with a razor blade as a reference point for positioning.
Figure 1. Schematic of Line Drawing on a 6-Well Plate
3) Incubation and Observation
Place the culture dish into a CO2 incubator for a period of time (usually 8-48 hours; shorter incubation times for faster migrating cells). After incubation, take images of the scratch area under a microscope.
4) Data Analysis
Compare the initial scratch distance with the final distance after incubation to calculate the migration rate:
Migration rate = (initial distance - final distance) / initial distance or (initial area - final area) / initial area.
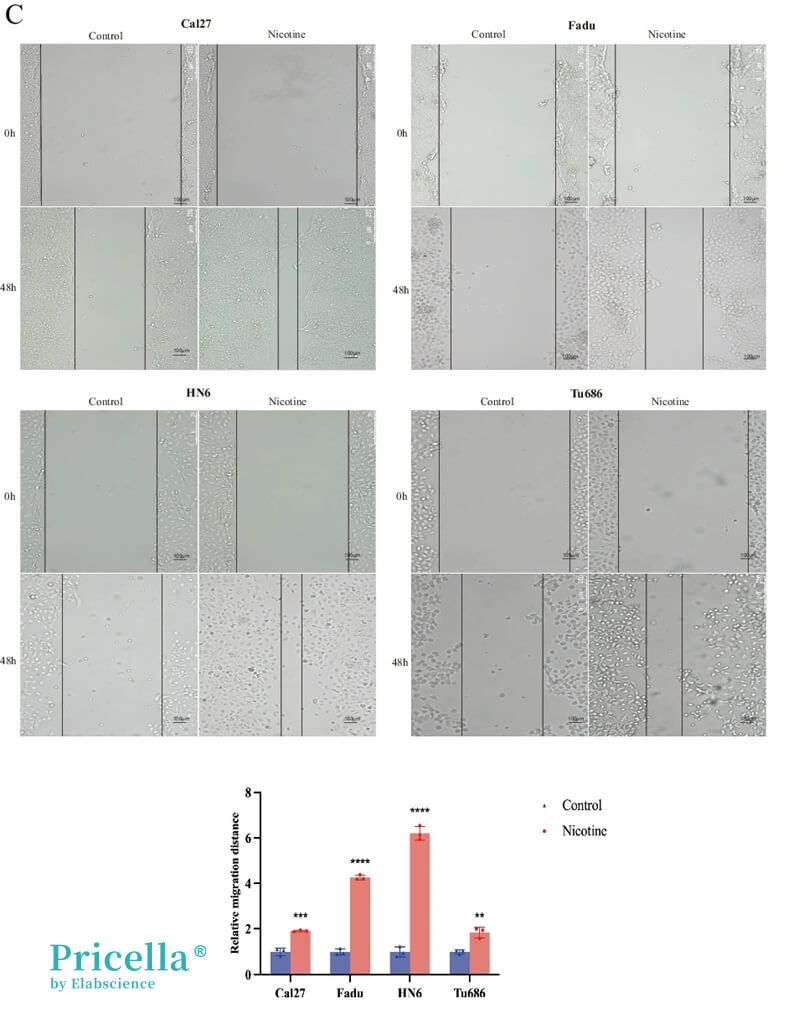
Figure 2. Cell scratch assay showing that exposure to 10 μmol/L nicotine significantly promotes cancer cell migration [2]
3. Key Considerations
• Cell Density Control
Cell density should neither be too low nor too high. If the density is too low, cells will have difficulty forming a monolayer, and gaps may form between cells, causing them to migrate in other directions rather than towards the scratch. If the density is too high, cells may be scraped off in chunks, resulting in irregular and uneven scratches.
• Consistent Scratch Width
Ensure that the scratch width is consistent across experimental and control groups to avoid errors caused by variations in scratch size.
• Incubation Time
Adjust incubation time based on cell type and migration speed to ensure scratch closure occurs under optimal conditions.
• Edge Cell Accumulation
It is recommended to perform the scratch assay in PBS and wash away excess cells around the scratch to prevent edge cell accumulation. [3]
• Consistent Imaging Location
Images must be taken from the same location to ensure comparability between pre- and post-migration photos.
• Proliferation Inhibition Treatment
Treat cells with mitomycin C or low-serum medium prior to the experiment to prevent cell proliferation from affecting migration results.
• Data Analysis
Each experiment should include at least three replicate wells, and it is recommended to record multiple fields of view per well to ensure rigorous and representative data.
References
[1] Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces [J]. Nature Reviews Cancer. 2017; 17(2):131-140.
[2] Feng C, Mao W, Yuan C, et al. Nicotine-induced CHRNA5 activation modulates CES1 expression, impacting head and neck squamous cell carcinoma recurrence and metastasis via MEK/ERK pathway [J].Cell Death & Disease. 2024 Oct 29; 15(10):785.
[3] Vang Mouritzen M, Jenssen H. Optimized Scratch Assay for In Vitro Testing of Cell Migration with an Automated Optical Camera [J]. Journal of Visualized Experiments Jove. 2018; (138):57691.
Prev: Cell Species Identification: Safeguarding the Pure Lineage of Research Cells
Next: Choosing the Right Transfection Method: Transient Transfection vs. Stable Transfection





