Troubleshooting Cell Aggregation in Culture
Source: PricellaPublished: 2025-03-14
Cell aggregation is a common and complex challenge in cell culture. It can significantly impact cell growth, morphology, and functionality, compromising the reliability of experimental results and posing challenges for researchers.
In this article, we explore the impact of cell aggregation on cell culture, analyze common causes of aggregation, and provide effective solutions to optimize culture conditions and enhance experimental success rates.
The Impact of Cell Aggregation on Cell Culture
01 Inhibited Cell Growth and Proliferation
Aggregation limits the exchange of nutrients and oxygen between cells, leading to uneven growth. Some cells proliferate vigorously, while others enter dormancy or face nutrient deprivation, potentially resulting in cell death.
02 Altered Cell Morphology and Functionality
Cells in aggregates often lose their normal adherence properties, negatively affecting key functions such as secretion and metabolic activity. In experiments requiring cell-to-matrix or cell-to-cell interactions, the presence of aggregates can disrupt these biological processes.
03 Reduced Reliability of Experimental Results
Aggregation can cause variability in cell behavior, reducing the reproducibility of experimental outcomes. For example, in drug screening or functional studies, aggregated cells may exhibit inconsistent drug responses, leading to false-positive or false-negative results, ultimately compromising the accuracy of research conclusions.
04 Complications in Subsequent Experimental Procedures
During passaging, aggregated cells increase the complexity of handling and the risk of damage, which can elevate cell mortality rates and impair the overall quality of the culture.
Common Causes of Cell Aggregation and Solutions
• Intrinsic Cell Characteristics
Some suspension cell lines naturally grow in aggregated forms due to their inherent properties. For instance, AtT-20 cells naturally grow in large clusters during suspension culture (Figure 1), U2932 cells typically grow in smaller aggregates (Figure 2). For such cell types, it is advisable to refer to authoritative cell line databases to familiarize yourself with their normal growth patterns. If aggregation is part of their natural behavior, no special intervention is required.
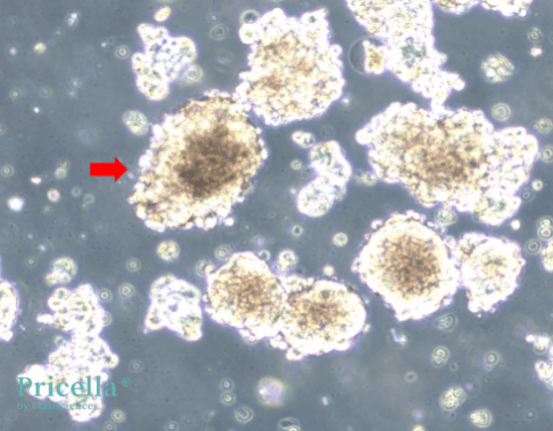
Figure 1: AtT-20 cells growing in large clusters
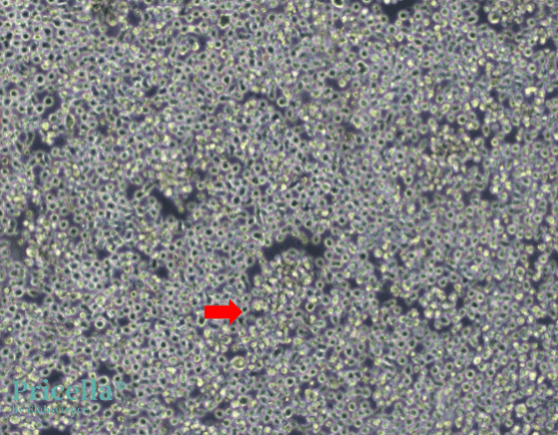
Figure 2: U2932 cells growing in small clusters
Additionally, suspension-adapted cell lines used in serum-free cultures (e.g., HEK 293F and CHO-S) are prone to aggregation at high densities (Figure 3). Aggregation at high densities can result in premature cell death, significantly reducing protein expression yields. To address this, add anti-clumping agents to the culture medium can effectively reduce aggregation (Figure 4), extend cell viability under high-density conditions, and enhance protein expression.

Figure 3: CHO-S cells without anti-clumping agent
Figure 4: CHO-S cells with anti-clumping agent
• Cellular Stress
Adherent cells with weak attachment properties (e.g., HEK 293 series) can detach and aggregate when exposed to external stress, such as introducing non-preheated culture medium during media changes, using PBS at non-optimal temperatures, subjecting cells to mechanical agitation.
Similarly, cells shipped at ambient temperature may detach and aggregate upon arrival. To resolve this, you can collect the aggregated cells, dissociate the aggregates with appropriate enzymes, and then re-seed the cells into fresh culture vessels.
For example, dorsal root ganglion (DRG) neurons, which are adherent cells, can aggregate and detach due to transport-induced stress (Figure 5). Under high magnification, the cell aggregates appear translucent and smooth. If the viability of aggregated cells is uncertain, dissociate the aggregates into single-cell suspensions and use trypan blue staining to assess viability. After re-plating and 24 hours of culture, cells will begin to reattach and spread out. Within 2 days, normal morphology is restored.
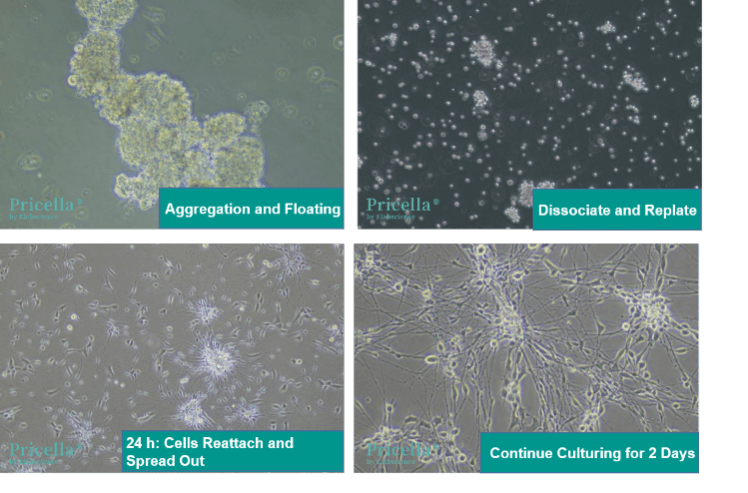
Figure 5: DRG neurons after ambient temperature shipping and re-plating steps
• Improper Dissociation
Inappropriate dissociation during passaging of adherent cells is closely related to post-passage aggregation. Over- dissociation (e.g., using high-concentration enzymes or excessively long dissociation times) can damage cells, impairing their adhesion properties and increasing the likelihood of aggregation. Conversely, under- dissociation may cause large cell sheets to detach from the culture surface without proper dissociation, making it difficult to create a single-cell suspension even with gentle pipetting.
To address this, carefully control the dissociation time to ensure thorough dissociation without compromising cell viability. If aggregation occurs post-passaging and cell viability is satisfactory, re-dissociate the cells to obtain a single-cell suspension before re-seeding.
• Serum Variability
Serum variability, including differences in growth factor content between brands or batches, can influence cell adhesion and growth, potentially triggering aggregation. Even within the same brand, batch-to-batch inconsistencies may lead to variations in cell behavior. To minimize aggregation risks, avoid switching serum brands or batches whenever possible. If a change is necessary, gradually transition to the new serum through incremental mixing with the current serum. This method improves the success rate of serum replacement and reduces aggregation risks during cell adaptation.
Prev: Master These Techniques to Distinguish Live and Dead Cells
Next: Exosome Isolation Guide: Comparing Five Key Methods to Maximize Your Efficiency





