Exosome Isolation Guide: Comparing Five Key Methods to Maximize Your Efficiency
Source: PricellaPublished: 2025-03-17
Exosomes are a subset of extracellular vesicles (EVs) ranging from 30 to 150 nm in diameter. Secreted by various cell types under both physiological and pathological conditions, they play a vital role in intercellular communication. Exosomes have attracted increasing attention in areas such as tumor microenvironment regulation, immune modulation, and neurodegenerative disease research [1].
As exosome research continues to evolve, the need for efficient and reliable isolation methods has become a priority. Each isolation technique differs in terms of purity, yield, and ease of use, making it critical to select the method that best aligns with your experimental goals.
In this guide, we break down five widely used exosome isolation meth ods, offering key insights to help you determine the most suitable approach for your research.
Choosing the Right Exosome Isolation Method
Method | Purity | Yield | Equipment Requirement | Best Use Case |
Ultracentrifugation (UC) | High | Low | High | Classic method for routine exosome studies |
Density Gradient Centrifugation (DGC) | Highest | Low | High | Ideal for ultra-pure exosome research |
Size-Exclusion Chromatography (SEC) | Medium | High | Low | For studies requiring intact exosomes |
Polymer Precipitation | Low | High | Low | Fast isolation for large-volume samples |
Immunoaffinity Isolation | Highest | Low | Low | Targeted research requiring high selectivity |
Detailed Overview of Exosome Isolation Methods
1. Ultracentrifugation (UC)
Principle: UC relies on size and density differences between exosomes and other components in the sample. A series of high-speed centrifugation steps progressively remove unwanted particles, resulting in a purified exosome pellet.
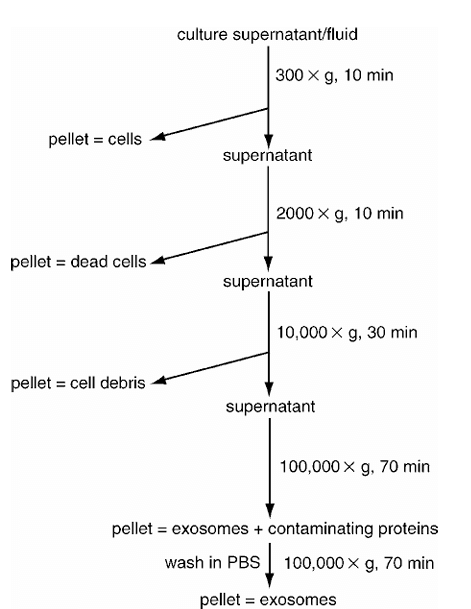
Figure 1. Schematic diagram of the ultracentrifugation process [2]
Advantages:
• Removes most contaminants, yielding relatively pure exosomes.
• Suitable for large-volume samples (e.g., cell culture supernatants, serum).
• No additional reagents required, avoiding chemical contamination.
Disadvantages:
• Requires an ultracentrifuge, which is expensive and complex to operate.
• High centrifugal forces may damage exosome integrity.
• Low recovery rate; some exosomes may remain in suspension.
2. Density Gradient Centrifugation (DGC)
Principle: DGC separates exosomes based on buoyant density differences using sucrose or iodixanol gradient media. Exosomes accumulate at their characteristic density range (1.13-1.19 g/mL) after ultracentrifugation.
Steps:
a) Pre-treatment: Remove cell debris and large particles.
b) Density Gradient Preparation: Create a layered sucrose or iodixanol gradient.
c) Ultracentrifugation: Load the sample and centrifuge at 100,000-120,000 ×g for 16-18 h at 4°C.
d) Exosome Collection: Extract the exosome-enriched fraction from the density range.
e) Purification: Resuspend in PBS and perform a final centrifugation step.
Advantages:
• Achieves high exosome purity by removing proteins and lipids.
• Ideal for experiments requiring ultra-pure exosomes.
Disadvantages:
•
Tedious and time-consuming (>16 h).
• Low sample throughput; not suitable for large-scale isolation.
3. Size-Exclusion Chromatography (SEC)
Principle: SEC utilizes porous beads in a chromatography column to separate molecules by size. Exosomes elute at specific fractions, while smaller proteins and other contaminants are excluded.
Steps:
a) Sample Preparation: Remove large debris and concentrate exosomes.
b) Column Setup: Pack SEC beads and equilibrate with buffer.
c) Sample Loading: Apply the sample carefully.
d) Fraction Collection: Collect exosome-enriched fractions.
e) Purification (Optional): Further concentrate exosomes if needed.
Advantages:
• Gentle, preserving exosome structure.
• Simple workflow without ultracentrifugation.
• Efficient removal of protein contaminants.
Disadvantages:
• Limited purity; may still contain other EVs.
• Not ideal for large-volume processing.
4. Polymer Precipitation
Principle: Polymers such as polyethylene glycol (PEG) reduce the solubility of exosomes, facilitating their precipitation at low centrifugal forces.
Steps:
a) Pre-treatment: Remove large debris.
b) Polymer Addition: Mix sample with precipitation solution and incubate.
c) Centrifugation: Collect the exosome pellet.
d) Resuspension and Purification: Re-dissolve in buffer and optionally purify further.
Advantages:
• Simple and fast (<4 h).
• No need for expensive equipment.
• Suitable for large-volume samples (serum, urine, etc.).
Disadvantages:
• Possible polymer contamination affecting downstream analysis.
• Low purity due to co-precipitation of proteins and other particles.
5. Immunoaffinity-Based Isolation
Principle: Uses antibody-coated magnetic beads or affinity resins targeting exosome surface markers (e.g., CD9, CD63, CD81) for selective capture.
Steps:
a) Pre-treatment: Remove large debris.
b) Antibody Coupling: Bind antibodies to solid-phase carriers.
c) Exosome Capture: Incubate the sample to allow specific binding.
d) Washing and Elution: Remove unbound material and elute exosomes.
Advantages:
• Extremely high purity.
• Enables specific isolation of exosome subpopulations.
• Ideal for sensitive downstream analyses (e.g., qPCR, Western blot).
Disadvantages:
• Expensive reagents and limited scalability.
• Lower recovery rates due to selective capture.
Future Outlook: Emerging Technologies in Exosome Isolation
Innovative approaches such as microfluidics and nanotechnology are revolutionizing exosome isolation. Microfluidic devices integrate size-based filtration with immunoaffinity capture, enabling high-throughput, low-input, and high-purity exosome isolation [3]. These advances hold great promise for biomarker discovery, cancer diagnostics, and therapeutic applications.
To overcome the challenges of expensive equipment and complex workflows, Pricella® offers five exosome isolation kits, including magnetic bead-based, SEC, and precipitation-based methods. These solutions streamline exosome extraction, empowering your research with efficiency and reliability.
References
[1] Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function [J]. Nature Reviews Immunology, 2002 Aug; 2(8):569-579.
[2] Théry C, Amigorena S, Raposo G, et al. Isolation andcharacterization of exosomes from cell culture supernatants and biological fluids [J]. Current Protocols in Cell Biolog, 2006 Apr; Chapter 3: Unit 3.22.
[3] Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine [J]. Lab on a Chip, 2017 Oct 25; 17(21):3558-3577.
Prev: Troubleshooting Cell Aggregation in Culture
Next: Is Slow Cell Growth an Abnormal Condition? How to Adjust it Properly?





