Key Considerations for Culturing NK-92 and and NK-92MI Cells
Source: PricellaPublished: 2025-01-03
Key Considerations for Culturing NK-92 and and NK-92MI Cells
• NK-92 cells are an IL-2-dependent natural killer (NK) cell line derived from human peripheral blood mononuclear cells.
• NK-92MI cells are an IL-2-independent NK cell line derived from NK-92 cells.
The parental NK-92 cells were genetically modified using retroviral-mediated transduction with the MFG-hIL-2 vector carrying human IL-2 cDNA.
As homologous cell lines, NK-92 and NK-92MI share many similarities, but their culture methods and handling precautions differ in some aspects.
In this article, we provide a detailed explanation of the key considerations for culturing NK-92 and NK-92MI cells.
1. Key Considerations for Cell Culture
Although NK-92 and NK-92MI cells are homologous, differences in IL-2 dependence require slight variations in their culture methods. Follow these guidelines:
• Both NK-92 and NK-92MI cells grow in suspension, forming clusters with some dispersed single cells. Dead cells and cell debris are often visible between the clusters.
• NK-92 cells are highly dependent on IL-2 degradation. A lack of IL-2 can result in poor cell condition. To prevent this: 1) Store IL-2 at -20°C, and transfer it to 4°C after thawing. Do not store IL-2 at 4°C for more than 2 weeks, 2) Use fresh culture medium promptly after preparation. Pre-warm the medium to room temperature before use, but avoid warming it in the incubator. Return unused IL-2 to the 4°C refrigerator promptly, 3) If NK-92 cells exhibit poor growth, an increased number of dispersed cells, or a lack of proliferation, supplement the culture with 200 U/mL IL-2 and culture for an additional 2–3 days to restore cell health, 4) NK-92MI cells are IL-2-independent but may also benefit from IL-2 supplementation at 200 U/mL if cell conditions deteriorate.
• Both NK-92 and NK-92MI cells are centrifugation-sensitive. To reduce stress: 1) Change the medium every 2–3 days, alternating between partial medium changes and complete medium changes by centrifugation, 2) Limit the frequency of centrifugation, especially when cells are in poor condition or at low densities.
Methods for Medium Changes:
▶ Medium Supplementation Method: Add fresh medium every 2–3 days based on the culture volume and container size (e.g., 1–2 mL for a T25 flask). For NK-92 cells, add 200 U/mL IL-2 to the medium. Repeat this process 2–3 times, then perform a complete medium change.
▶ Partial Medium Change Method:
• Stand the flask upright and let the cells settle at the bottom. Before standing, lightly tap the flask to loosen any cells adhering to the bottom.
• Once the cells have settled (visible to the naked eye), carefully aspirate half of the medium (e.g., 2.5 mL from a T25 flask) and transfer it to a centrifuge tube.
• Centrifuge the medium at 1000 rpm for 3–5 minutes. Resuspend the pellet in fresh medium and return it to the flask.
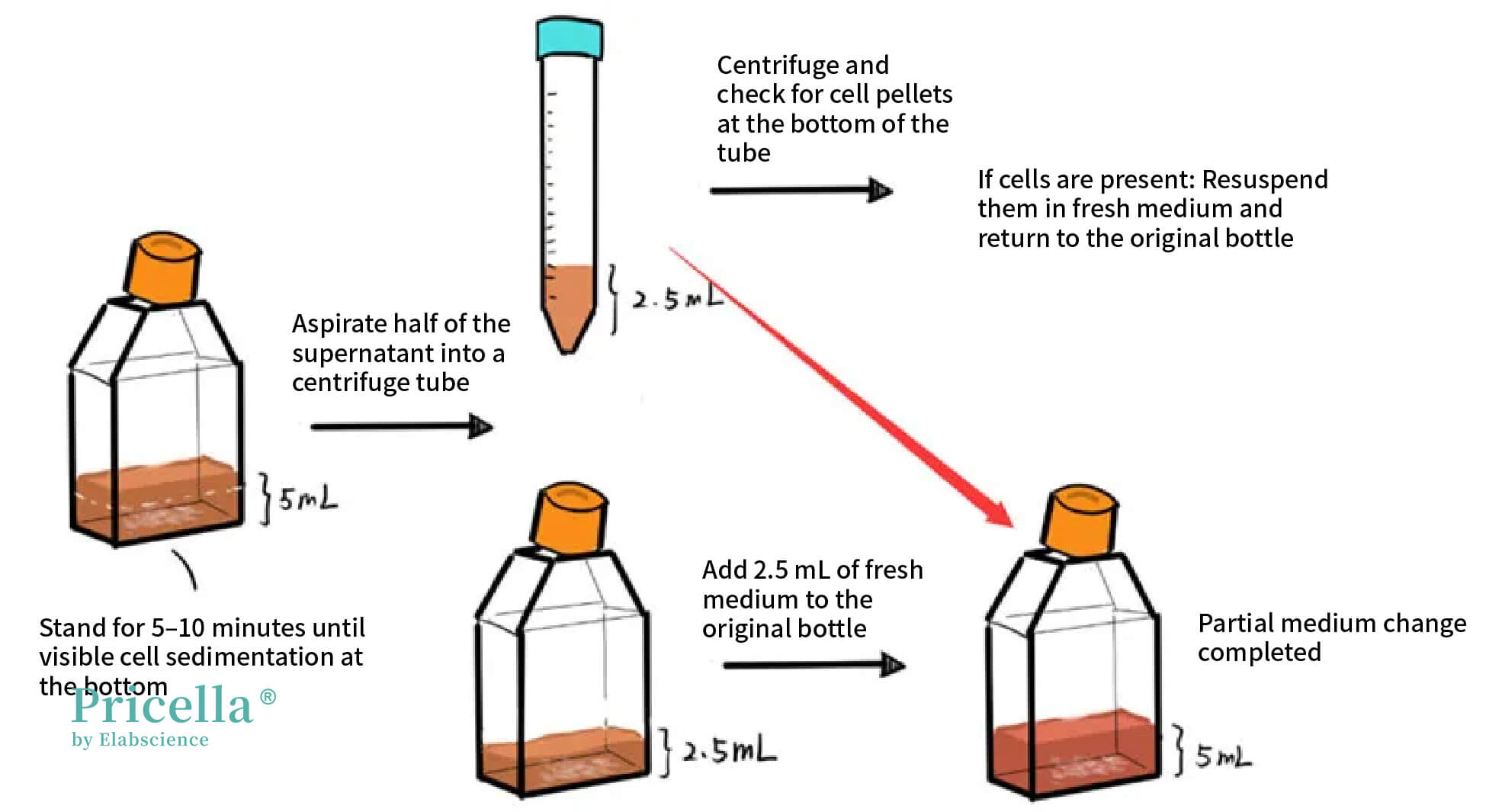
Figure 1: Partial Medium Change Method
As cell clusters grow, they may become too large, leading to insufficient nutrient availability in the inner regions of the clusters. When this occurs:
Subculturing
▶ Gently dissociate the clusters using a pipette. Pipette 2–3 times gently to avoid generating excessive cell debris or dead cells. Split the cells equally into two flasks and add fresh medium to each flask.
Avoid allowing clusters to grow too large, as this may lead to nutrient deprivation. Clusters require only need slight dissociation; complete dissociation into single cells is unnecessary.
1. Cryopreservation
• Use a freezing medium consisting of 90% FBS and 10% DMSO. Supplement the freezing medium with IL-2 for NK-92 cells, however,this is unnecessary for NK-92MI cells.
• Before freezing, gently dissociate the cell clusters to ensure even distribution and minimize clumping.
2. Thawing and Recovery
The thawing and recovery process is identical for NK-92 and NK-92MI cells. Follow these steps:
• After thawing, resuspend the cells in 5 mL of fresh culture medium.
• Stand the flask upright (with the cap loosened) in the incubator to increase local cell density.
• Observe the cells once daily. Avoid frequent handling or removing the flask from the incubator.
• Add fresh medium every 3 days (1–2 mL per addition). After 1–2 supplementation cycles, perform a partial medium change. Avoid frequent centrifugation.
• Once the medium turns yellow and clusters grow significantly larger, subculture into a new flask. Do not exceed a 1:1 split ratio.
3. Dissociating Cell Clusters
• Minimize centrifugation and control the number of pipetting cycles during subculturing. Clusters should be dissociated but not completely broken down into single cells.
• After subculturing or medium changes, clusters will naturally reform within 1–2 days.
• If clusters become too large, dissociate them slightly to improve nutrient access.
• During cryopreservation, ensure clusters are fully dissociated to improve freezing and recovery efficiency.
Prev: Does BEAS-2B Require Serum for Culture?
Next: Why Human Chondrocytes Morphologically Change After Passaging?





