Five Common Causes of Cell Elongation and How to Address Them
Source: PricellaPublished: 2025-02-19
Under normal in vitro culture conditions, cells typically exhibit flat, polygonal, or round shapes. These morphologies are essential for critical physiological functions, such as nutrient absorption, proliferation, and intracellular transport. However, many researchers encounter issues with abnormal cell morphology during culture, particularly cell elongation. This phenomenon can indicate declining cell health and may significantly impact experimental accuracy and progress.
In this article, we will explore the common causes of cell elongation during culture and provide targeted solutions to help you maintain healthy, thriving cells.
Common Causes of Cell Elongation and Solutions
• Elongation as a Normal Morphological Trait
Some cell lines naturally exhibit elongated or thread-like morphologies during routine culture. For example, SH-SY5Y (human neuroblastoma cells) and K7M2 wt (mouse osteosarcoma osteoblast cells) often display stretched or antenna-like features under a light microscope, as shown in Figures 1 and 2. This is part of their normal morphology and does not indicate a problem.
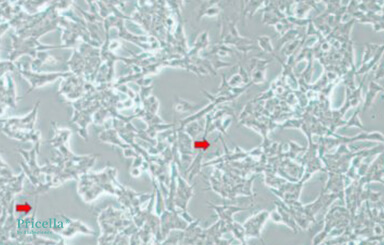
Figure 1. Morphology of SH-SY5Y cells
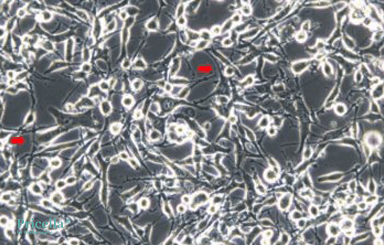
Figure 2. Morphology of K7M2 wt cells
Solution:
If you observe elongation during the initial culture of an unfamiliar cell line, consult images or documentation provided by reputable cell banks to determine whether this is a natural feature of the cell line. For cells with inherent elongation characteristics, no special intervention is required. However, if the elongation is accompanied by slower proliferation or other abnormalities and does not match the expected morphology, further investigation is warranted.
• Suboptimal Seeding Density
Improper seeding density can lead to cell elongation. Overly high cell density increases competition for nutrients and space, resulting in morphological stress and elongation. Conversely, overly low density, especially when using serum-free media for culturing mesenchymal stem cells, may inhibit proper cell growth and lead to spindle-like elongation.
Solution:
Adjust seeding density based on the specific requirements of the cell line. Maintain an appropriate range of cell density through regular passaging to prevent overcrowding or under-seeding.
• Inappropriate Culture Conditions
Culture conditions such as incorrect media formulations, insufficient key nutrients (e.g., amino acids, vitamins, and growth factors), pH imbalances, temperature fluctuations, or unsuitable gas conditions (e.g., CO₂ concentration) can disrupt normal cell morphology and cause elongation.
Solution:
Use freshly prepared media and replenish nutrients as needed by performing timely media changes. Ensure the culture environment is stable, with proper pH, temperature, and gas levels. If needed, increase serum concentration (not exceeding 20%) or switch to high-quality fetal bovine serum (FBS), which can improve cell morphology (Figure 3).
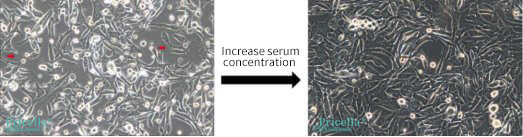
Figure 3. Morphology of Renca cells (mouse renal carcinoma cells)
• Improper Handling During Passaging
Handling errors during passaging, particularly for adherent cells, can cause significant cell damage and lead to morphological changes like elongation. Using the wrong dissociation reagent, over-dissociating, or applying excessive mechanical force during detachment can all result in abnormal cell morphology.
Solution:
Choose appropriate dissociation reagents for the specific cell type. Carefully monitor dissociation times to avoid overexposure to enzymes. Handle cells gently during detachment to minimize damage and morphological stress.
• Contamination
Contamination from bacteria, fungi, mycoplasma, or nanobacteria can severely affect cell health by competing for nutrients and releasing cytotoxic metabolic byproducts. These conditions can significantly degrade cell status and lead to elongation or other abnormal morphologies.
Solution:
Implement strict aseptic techniques, including the use of sterile reagents and consumables. Regularly screen for hard-to-detect contaminants like mycoplasma using PCR-based assays or by conducting empty culture (media-only) tests. If contamination is identified, address it promptly to prevent further spread and damage to cell cultures.





