C2C12 Cell Cultivation Strategy and Differentiation Protocol - Pricella
Source: PricellaPublished: 2024-12-25
C2C12 (mouse myoblasts) is a subclone of a mouse myoblast cell line established by D. Yaffe and O. Saxel. C2C12 cells differentiate rapidly, readily forming contractile myotubes and generating characteristic muscle proteins. When treated with bone morphogenetic protein 2 (BMP-2), the differentiation pathway of C2C12 cells shifts from myogenesis to osteogenesis.
As an ideal model for muscle research, C2C12 cells are often induced to differentiate into multinucleated myotubes in experimental studies. Myogenic differentiation plays a crucial role in muscle regeneration and occurs through a highly coordinated sequence of events leading to the formation of mature skeletal muscle.
Basic Information:
Growth Characteristics: Adherent cells
Cell Morphology: Myoblast-like
Culture Medium: DMEM + 10% FBS + 1% P/S
Recommended Subcultivation Ratio: 1:3-1:4
Recommended Medium Change Frequency: 2-3 times per week
Doubling Time: ~20 hours
Cryopreservation Conditions:
Freezing Medium: 55% basal medium + 40% FBS + 5% DMSO
Temperature: Liquid nitrogen
Culture Conditions
Atmosphere: 95% air, 5% CO2
Temperature: 37°C
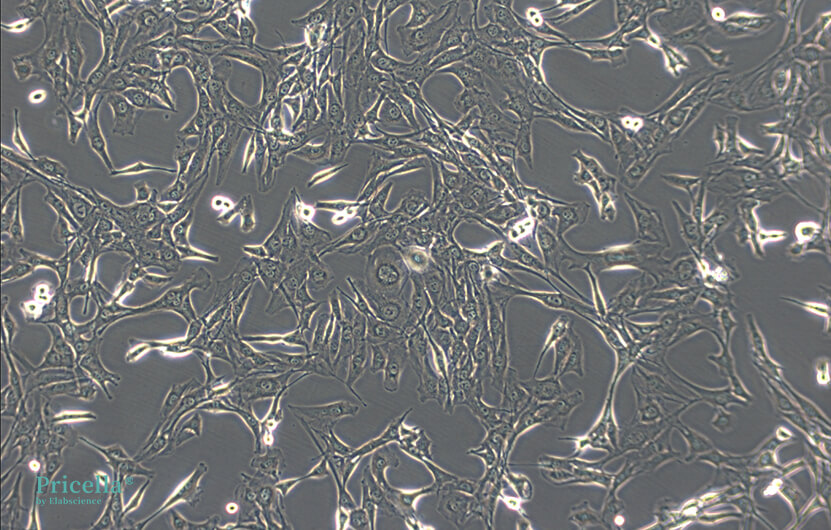
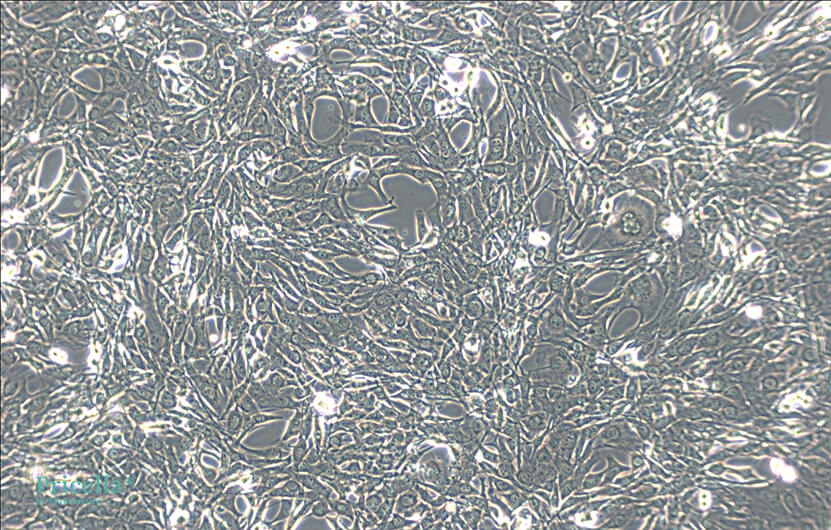
The state of C2C12 cells directly impacts their differentiation potential. To achieve optimal differentiation, meticulous attention must be given to cell culture. The medium replacement and subcultivation steps follow standard procedures for adherent cells. Additionally, note the following three points:
1. Fast Growth and Easy Differentiation: Subculture when the density reaches 70%.
2. Avoid Overconfluence: Do not allow cells to become overly confluent or start to fuse, as this can negatively affect subsequent myogenic differentiation rates.
3. Complete Digestion: Ensure cells are fully digested before pipetting to avoid clumping.
4. When C2C12 cells reach approximately 80% confluence, differentiation can be induced. The specific steps are as follows.
Differentiation Protocol:
1. Discard the old culture medium and wash twice with PBS.
2. Change to differentiation medium (high glucose DMEM + 2%-6% horse serum + 1% P/S) for induction, then incubate in a CO2 incubator.
*Note: Due to variability in horse serum quality, it is recommended to test concentrations at 2%, 4%, and 6% to determine the optimal concentration. Change the medium every 24 hours and observe cell morphology and growth under a microscope. Successful induction of differentiation will be evident by the appearance of thick, tubular structures, sometimes multinucleated.
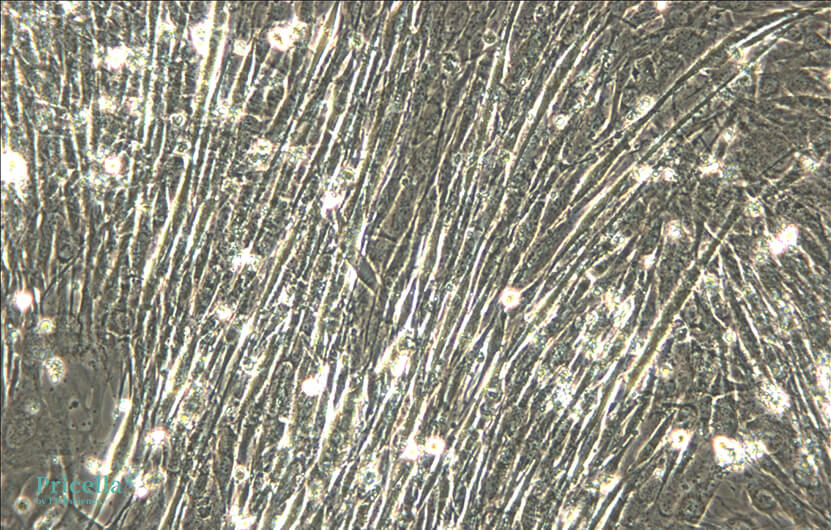
Tips:
Avoid using C2C12 cells at high passage numbers.
If cells continue to proliferate after switching to differentiation medium, use Giemsa staining to verify the presence of differentiated myofibers.
A small amount of apoptosis during C2C12 myogenic differentiation is normal.
This guide provides the fundamental steps and considerations for culturing and differentiating C2C12 cells, ensuring a professional and effective approach to cell culture research.
Prev: A Deep Dive into the HEK293 Cell Line Family
Next: Morphology, Growth, and Troubleshooting in BV2 Cell Culture





