A Detailed Guide to 3T3-L1 Adipogenic Differentiation
Source: PricellaPublished: 2025-01-16
The 3T3-L1 cell line, a mouse embryonic fibroblast cell line, was first isolated by Green and Kehinde in 1974. Due to its ability to differentiate into adipocyte-like cells in vitro, it has been widely used in the study of metabolic diseases such as obesity and diabetes.
This article provides a step-by-step guide for 3T3-L1 adipogenic differentiation, including important tips to help you master the experimental techniques and ensure successful results.
The following experiment was conducted using P5 passage cells for differentiation induction. For optimal accuracy and reliability, we recommend using early-passage cells for adipogenic differentiation experiments.
Note: This 3T3-L1 adipogenic differentiation protocol is based on internal test data from Pricella and is for reference only.
3T3-L1 Adipogenic Differentiation Steps
(Example for a 6-well plate)
1 Culture 3T3-L1 cells under normal conditions until cell confluency reaches 80–90%, then digest with trypsin and count the cells.
2 Seed the cells at a density of 2–3 × 10⁴ cells/cm² in a 6-well plate. Add 2 mL of complete 3T3-L1 culture complete medium per well. Adjust the number of seeded wells based on pre-experimental conditions.
3 Place the 6-well plate in a 37°C, 5% CO₂ incubator and allow the cells to grow evenly.
4 When cell confluency reaches 90–95%, carefully remove the complete medium and add 2 mL of 3T3-L1 Adipogenic Differentiation Medium A per well to initiate differentiation.
5 After 2 days in Medium A, remove it and add 2 mL of 3T3-L1 Adipogenic Differentiation Medium B for 1 day of maintenance culture.
6 Continue alternating 2 days in Medium A and 1 day in Medium B to maintain differentiation.
7 Follow the instructions in the 3T3-L1 adipogenic differentiation medium manual to prepare the Oil Red O staining solution.
Results of Induction
Before Induction: At 90–95% confluency, the 3T3-L1 cells are ready for differentiation.
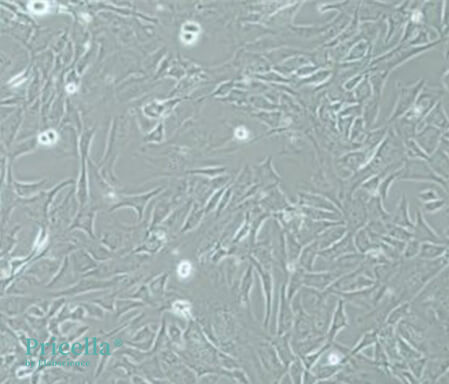
Microscopic Image Before Induction
Day 8 of Induction (Unstained): Using the 3T3-L1 Adipogenic Differentiation Medium, results on Day 8 are as follows
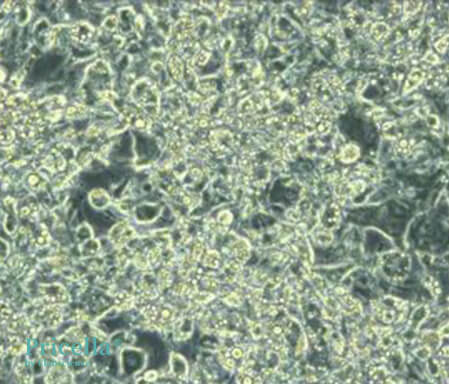
Unstained Image on Day 8
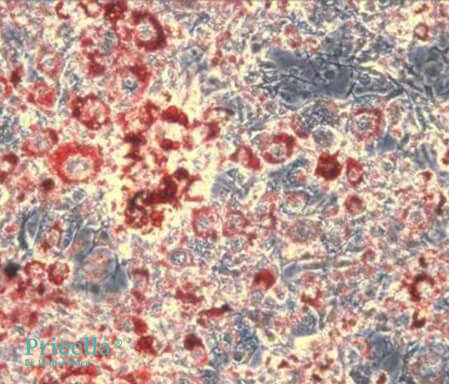
Day 8 of Induction (Oil Red O Staining)
Day 16 of Induction (Optional for Larger Lipid Droplets): To increase the size and quantity of lipid droplets, extend the induction period. Results on Day 16 are as follows
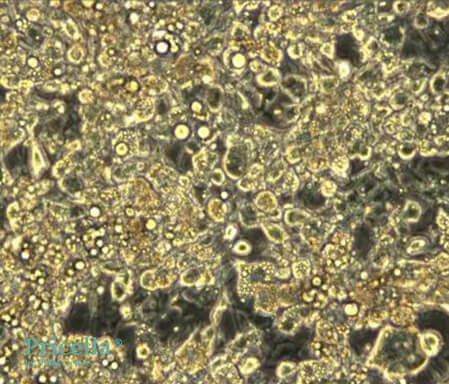
Unstained Image on Day 16
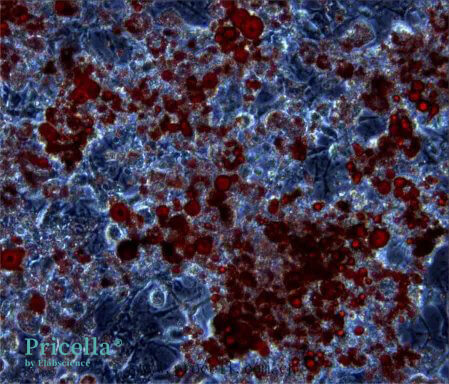
Oil Red O Stained Image on Day 16
Key Considerations for Induction
• Cell Density: Begin induction when cell confluency is at 90–95% or fully confluent. Avoid over-confluent cells, as they may detach, curl at the edges, or exhibit poor morphology, affecting differentiation results.
• Even Seeding: Uneven seeding can lead to inconsistent differentiation and increased cell detachment during induction.
• Duration: The induction process generally takes about 2 weeks; however, adjust the duration based on the appearance of lipid droplets.
• Handling Induction Medium: Add the differentiation medium gently. Pre-warm the reagents to 37°C before use. Pipette the medium along the wall of the well to minimize disruption, as forceful handling can dislodge cells.
• Fresh Solutions: Always prepare solvents and reagents before use.
• Oil Red O Staining: Dilute the Oil Red O staining solution according to the manual. Saturated Oil Red O solutions are less effective for staining.
Prev: Mastering Osteogenic Induction of MC3T3-E1 Subclone 14 Cells





