A Comprehensive Overview of A-498 Cells
Source: PricellaPublished: 2024-04-22
A-498 (human renal carcinoma cells) cell line was established by Aaronson·S. It is derived from the renal tissue of a 52-year-old female patient with renal carcinoma, exhibiting epithelial morphology. This cell line serves as a suitable transfection host and holds significant application value in tumor research, often employed as a model for renal cell carcinoma studies.
In this issue, we have compiled the cultivation conditions, passaging, cryopreservation steps, and common applications of A-498 cells to help you quickly get started with your experiments.
Cell Morphology
A-498 cells adhere to the substrate and exhibit epithelial-like morphology. They spread out extensively, with a relatively low total cell count per unit area, and some cells may display filopodia.
At low density, individual cells exhibit clear boundaries, while at densities exceeding 100%, the boundaries become less distinct, and there is no space between neighboring cells.
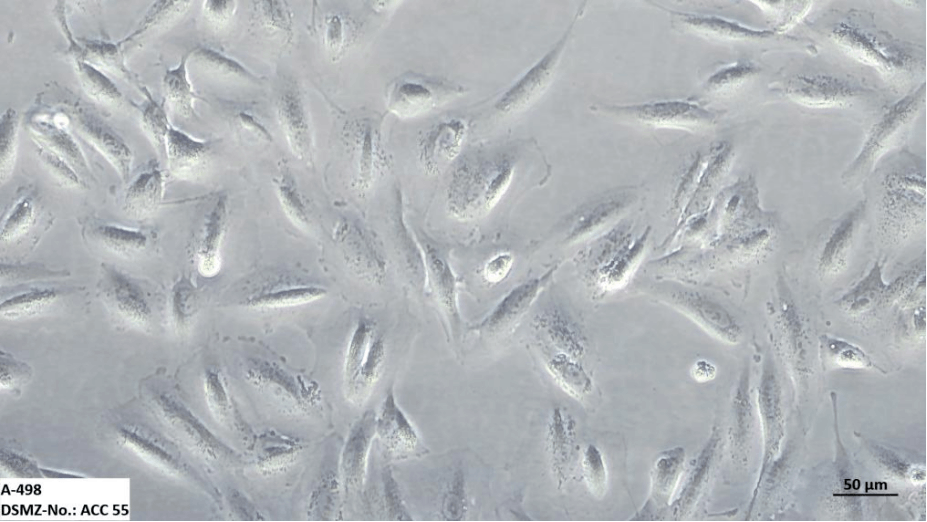

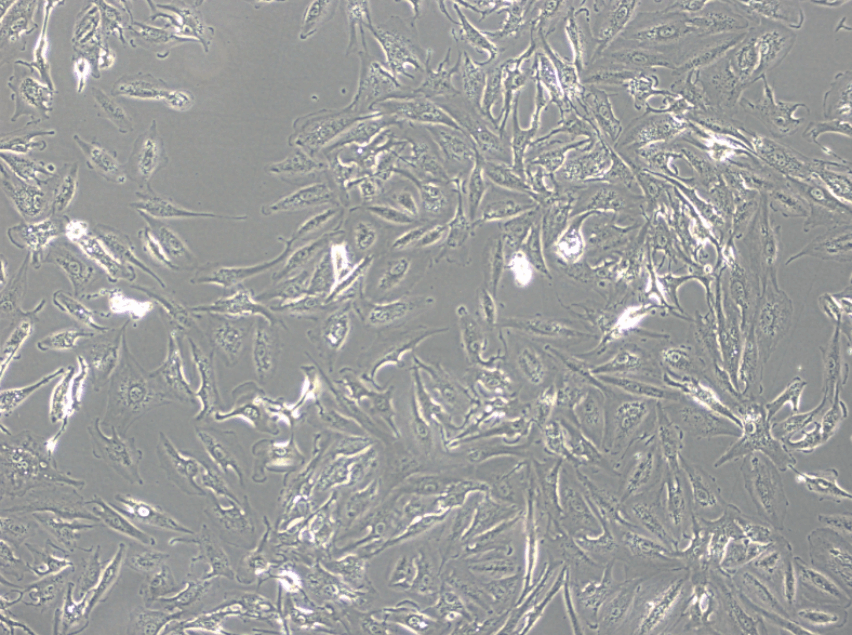
A-498 Cell Microscopic Images
Culture Method
● Culture Conditions
Culture Medium: A-498 Cells Complete Medium (Cat.No.: CM-0254);
Gas Phase: Air, 95%; Carbon Dioxide, 5%;
Temperature: 37°C;
Humidity: 70%-80%
● Growth Characteristics
Adherent Growth, Epithelial-like
● Doubling Time
33-36h;
● Cryopreservation Conditions
Cryopreservation Medium: 55% Basal Culture Medium + 40% FBS + 5% DMSO
Temperature: Liquid Nitrogen
Passaging of A-498 Cells:
1 When the cell density reaches 80%-90%, it is ready for passaging.
2 Discard the supernatant and rinse the cells with PBS (calcium- and magnesium-free) 1-2 times.
3 Add 1 mL of trypsin (containing 0.25% EDTA) (Cat.No.: PB180225) to the culture vessel and place it in a 37°C incubator for 2-3 minutes to digest. Then, observe the cell detachment under a microscope. If most cells have rounded up, detached, and are ready to be harvested, quickly remove the vessel from the incubator, gently tap the vessel several times, and add a small amount of medium to stop the digestion.
4 Add 3-4 mL of medium per vessel, gently pipette up and down to mix, then aspirate the mixture. Centrifuge at 1000 rpm for 4 minutes, discard the supernatant, and resuspend the cell pellet in 2-3 mL of fresh medium.
5 For the first passage, it is recommended to seed the cells at a 1:2 ratio into new dishes or flasks containing fresh medium. The total volume of medium per T25 flask should be 5-6 mL after passaging. Subsequent passages can be performed at a ratio of 1:2 to 1:4, depending on the cell growth characteristics.
Precautions:
A-498 cells exhibit extensive spreading with a relatively low total cell count per unit area. It is essential to control the passaging density appropriately. We recommend passaging at a ratio of 1:2 to 1:4 when the cell density reaches 80%. Additionally, it is advisable to change the medium or perform passaging every 2-3 days.
Cryopreservation of A-498 Cells:
(with serum; gradual cooling required):
1 Prepare cryopreservation media. Recommended proportions: 55% (basal culture medium) + 40% (serum) + 5% (DMSO), or 90% fetal bovine serum + 10% DMSO.
2 Count the prepared cell suspension to determine the total cell count.
3 After centrifugation, carefully remove as much supernatant as possible. Centrifuge at 1200 rpm (250g) for 3 minutes.
4 Resuspend the cells in the prepared freezing solution, adjusting the cell concentration to 3×106 - 1×107 cells/mL.
5 Distribute into cryovials, with 1 - 1.5 mL per vial.
6 It is recommended to use a controlled-rate freezing container. Place the filled cryovials into the container and directly transfer to a -80°C freezer overnight.
7 Transfer the cryovials swiftly from the -80°C freezer to liquid nitrogen for long-term storage.
Common Applications
A-498 cells are frequently used as a model for renal cell carcinoma research:
1 Studying the effects of specific signaling pathways on cell proliferation and invasion by transfection.
2 Studying the inhibitory effects of drugs on the progression of renal carcinoma cells and screening for potential anticancer drugs.
3 Utilizing A-498 cells to create animal models to simulate the behavior of renal cell carcinoma cells in vivo, enabling research into the biological characteristics of renal cell carcinoma.
Reference:
● Yan Sun,Liang Zhu,et al.ZDHHC2-Mediated AGK Palmitoylation Activates AKT–mTOR Signaling to Reduce Sunitinib Sensitivity in Renal Cell Carcinoma. Cancer Res (2023) 83 (12): 2034–2051.https://doi.org/10.1158/0008-5472.CAN-22-3105
Prev: Addressing Four Common Challenges with Cell Culture Reagents





