Isolation and Extraction of Rat Bone Marrow Mesenchymal Stem Cells
Source: PricellaPublished: 2025-01-17
Bone marrow mesenchymal stem cells (BMSCs) are derived from the mesoderm and exhibit multipotent differentiation potential. Research in cell biology indicates that BMSCs possess a strong proliferative ability and can differentiate into mesoderm-derived lineages, such as adipocytes, osteoblasts, and myocytes under specific induction conditions. BMSCs are considered an ideal source of seed cells and hold significant clinical applications in cell replacement therapy, gene therapy, and tissue engineering.
This article provides a comprehensive guide to the isolation and extraction of rat BMSCs, including methods, precautions, and identification markers to efficiently help initiate your experiments.
Isolation and Extraction Procedures
• Bone Marrow Collection
Euthanize the rat by cervical dislocation and immerse the body in 75% ethanol for 5-10 minutes to sterilize. Transfer the rat to a biosafety cabinet. Sterilely isolate the femurs and tibias, ensuring minimal tissue contamination. Use clean scissors and forceps to remove the surrounding muscles and retain the intact bones. Transfer the bones to a fresh culture dish containing PBS. Remove the bone ends to expose the marrow cavity. Using a syringe, flush the bone marrow with culture medium until the bone appears white and translucent. Repeatedly aspirate and eject the contents to form a single-cell suspension.
• Separation Methods
The common methods for isolating BMSCs include density gradient centrifugation, the adherence method, and immunomagnetic bead sorting. You can choose the appropriate method based on the conditions of your laboratory.
1. Density Gradient Centrifugation
• Centrifuge the bone marrow suspension at 1,500 rpm for 5 minutes.
• Discard the supernatant and re-suspend the pellet in complete culture medium.
• Carefully layer the suspension onto a Percoll separation solution (diluted with PBS) to form a density gradient.
• Centrifuge at 2,000-2,500 rpm for 25 minutes.
• Aspirate the intermediate white, mist-like cell layer, wash the cells with PBS, and centrifuge at 1,800 rpm for 5 minutes.
• Discard the supernatant, re-suspend the cell pellet in complete medium, and seed the cells into T25 flasks at a density of 1×10⁶ cells/mL. Culture at 37°C, 5% CO₂ in an incubator.
• Change the medium after 2 days, discarding non-adherent cells. Refresh the medium every 2-3 days. Upon reaching 80% confluency, digest with trypsin for 1-2 minutes and passage the cells.
2. Adherence Method
Also known as the whole marrow culture method or direct method.
• Filter the collected bone marrow suspension through a 200-mesh sieve and prepare a single-cell suspension in complete medium.
• Change the medium after 48 hours, discarding non-adherent cells.
• Refresh the medium every 2-3 days. Monitor cell growth and passage the cells when confluency exceeds 80%.
3. Flow Cytometry or Immunomagnetic Bead Sorting
These methods identify and separate cells based on surface-specific antigens, yielding high-purity cells. However, they may reduce cell viability and result in limited yields. Specialized equipment is required, limiting their application.
Precautions
• Strict Aseptic Techniques: Replace instruments after isolating the rat's hind limbs and removing fur to avoid cross-contamination.
• Nutrient-Rich Culture Media: Optimize serum concentration and consider adding growth factors.
• Percoll Gradient: Prepare fresh Percoll separation solution. Maintain a suitable ratio of separation solution to cell suspension, typically 1:1.
• Cell Seeding Density and Medium Changes: Low seeding density affects adherence and confluency. Adjust medium changes and passage times based on cell growth status.
Identification Criteria
1. Morphological Observation
Newly isolated primary cells are round, variable in size, and float in the culture medium (Figure 1).
After purification and adherence, the cells exhibit a fibroblast-like morphology, tightly arranged in a whorled growth pattern, with large, elongated nuclei (Figure 2). These features are consistent with BMSCs.
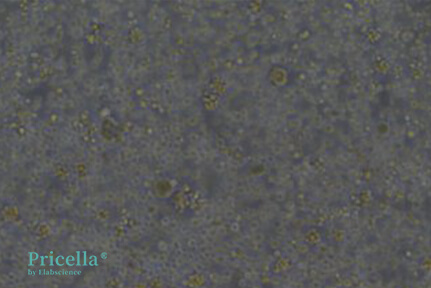
Figure 1: Newly isolated rat bone marrow mesenchymal stem cells (round morphology)
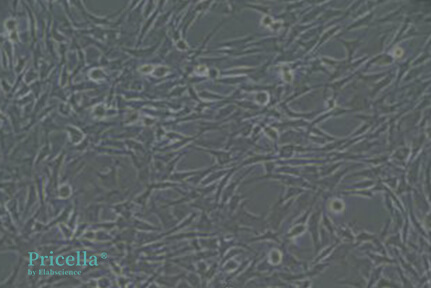
Figure 2: Purified rat BMSCs from Pricella Laboratory (fibroblast-like morphology)
2. Surface Markers
Commonly used markers include CD90, CD29, and CD44, which are expressed positively.
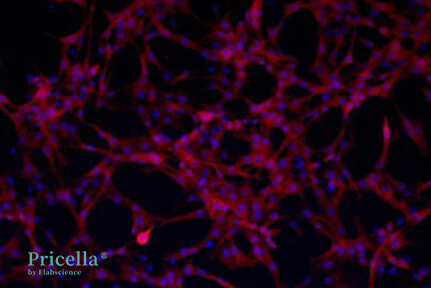
Figure 3: Expression of CD90 in rat BMSCs (Pricella Laboratory)
References:
[1]Clavin, N. W., Fernandez, J., Schönmeyr, B. H., Soares, M. A., et al. Fractionated doses of ionizing radiation confer protection to mesenchymal stem cell pluripotency. Plastic and reconstructive surgery, 2008; 122(3), 739-748. https://doi.org/10.1097/PRS.0b013e318180edaa
[2]Mushahary, D., Spittler, A., Kasper, C., et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry. Part A : the journal of the International Society for Analytical Cytology, 2018; 93(1), 19-31. https://doi.org/10.1002/cyto.a.23242[3]Li, X., Zhang, Y., & Qi, G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells.
Cytotechnology, 2013; 65(3), 323-334. https://doi.org/10.1007/s10616-012-9497-3
Prev: Common Issues in Cell Transfection
Next: Comprehensive Guide to Laboratory Contamination Management





