Culturing MEG-01 Cells: Tips and Considerations
Source: PricellaPublished: 2024-07-29
Basic Information
1 Cell Name
MEG-01 (Human Megakaryoblastic Leukemia Cell Line)
2 Aliases
Meg-01; MEG01; Meg01
3 Cell Culture Conditions
RPMI-1640+10% FBS+1% P/S,37℃;5% CO2
4 Growth Characteristics
Semi-adherent and semi-suspension
5 Cell Morphology
Lymphoblast-like
Background Information
MEG-01 is a megakaryoblastic cell line derived from a Philadelphia chromosome (Ph)-positive chronic myeloid leukemia (CML) patient. This cell line was established in 1983 by Michinori Ogura and Yasuo Morishima from the bone marrow sample of a 55-year-old male patient with CML in blast crisis.
Most MEG-01 cells in suspension are round or oval-shaped, while adherent cells extend pseudopodia. The cytoplasm is relatively basophilic and contains a few vacuoles. Cytoplasmic protrusions are also observed. Indirect immunofluorescence assays with monoclonal antibodies indicate that FVIII-related antigen is negative on the surface of MEG-01 cells but positive within the cytoplasm, especially in larger cells. All cells exhibit strong positive staining for acid phosphatase, which is diffuse and finely granular.
MEG-01 cells serve as a valuable model for studying human megakaryocyte differentiation and maturation. Additionally, they are useful for research into the production and release of platelets or proteins such as FVIII-related antigen, platelet glycoproteins, or platelet-derived growth factor (PDGF).
Cell Characteristics
During culture, some black dot-like debris is always present and does not require special treatment. Most cells exhibit an irregular round shape in suspension, with a few cells displaying a spindle-shaped adherent morphology.
Culture Methods
Using a T25 flask as an example:
1. MEG-01 cells are semi-adherent and semi-suspension cells. It is normal for adherent cells to be less than or up to 50%. These can be passaged as suspension cells by detaching the adherent cells (use 0.25% trypsin if cells do not detach easily).
2. Maintain cell density at 3-10 × 10^5 cells/mL. The initial seeding density should not be lower than 3 × 10^5 cells/mL. Passage the cells when they reach approximately 10 × 10^5 cells/mL. It is recommended to count the cells for the initial passages, and later passages can be based on experience.
3. Count the cells every 3 days. If the density is below 3 × 10^5 cells/mL, transfer to a smaller volume container (e.g., multi-well plate) for culture.
4. Methods Based on Cell Density:
Method 1: Partial Media Change
If the density is less than 8 × 10^5 cells/mL, perform a partial media change. Slowly remove half of the upper layer of the culture medium and centrifuge at 1200 rpm (approximately 250g) for 3 minutes to collect the cells. Resuspend the cells in an equal volume of fresh medium, pipette gently 3-5 times, and return to the original flask for continued culture.
Method 2: 1:2 Dilution Passage
If the density is close to 10 × 10^5 cells/mL, split the cells equally into two new culture flasks, and add fresh medium to a total volume of 5 mL per flask.
Method 3: Full Centrifugation and Media Change/Passage
For cultures extending beyond one week, perform a full centrifugation and media change or passage once weekly. Frequent centrifugation for media change or passage is not recommended. Centrifuge at 1200 rpm for 3 minutes.
Special Precautions
01 MEG-01 cells proliferate slowly and are relatively fragile. Minimize handling and maintain a stable culture environment and temperature.
02 Minimize pipetting. When sampling, counting, or passaging, gently pipette 3-5 times to mix the cells.
03 Observe cell status daily post-passage. Overcrowded cultures lead to cell death; hence, strictly control culture density.
04 Perform partial media changes or passages at least every 3 days and a full centrifugation and media change once a week.
Cryopreservation and Thawing
Recommended Freezing Density:
3 - 5×106 cells/mL ;
Freezing Medium
Use a controlled-rate programmed cooling box for gradual temperature decrease.
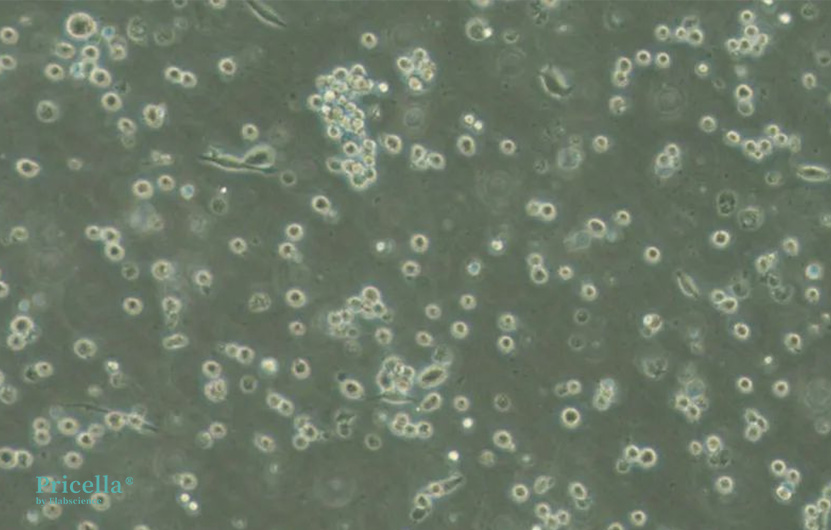
Figure 1: Culture image from Pricella.
Prev: Key Considerations for Culturing NCI-H520
Next: Optimizing 22RV1 Cell Growth: Detailed Protocols and Best Practices





