Comprehensive Guide for Handling Floating Neuronal Cells
Source: PricellaPublished: 2024-06-26
Neurons are cells characterized by long extensions called axons. Each neuron consists of a cell body and neuron projections. Axons are typically covered by a myelin sheath, forming nerve fibers, and their terminal branches are known as synaptic terminals.
The cell body of a neuron is located in the brain, spinal cord, and ganglia, while the neuron projections can extend to various organs and tissues throughout the body. These projections, originating from the cell body, are categorized into dendrites and axons. A neuron may have one or multiple dendrites, which receive signals and transmit them to the cell body. Conversely, each neuron has only one axon, which conveys signals from the cell body to another neuron or to other tissues such as muscles or glands.
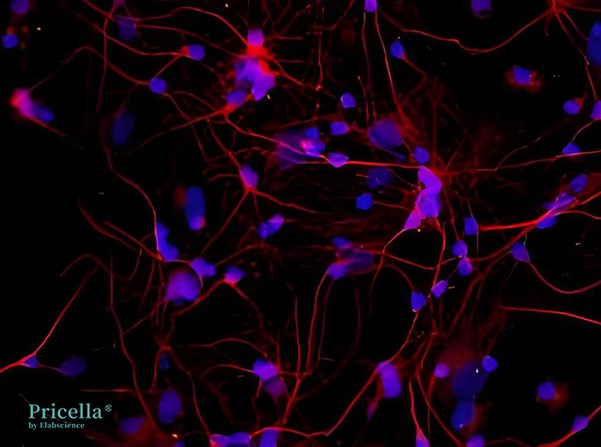
Figure 1. Rat Cortical Neurons
Neuronal cells are relatively delicate, and upon receipt, they may appear to be floating.
Today, Pricella® will guide you on how to handle floating cells upon receipt and provide important culture tips to ensure your experiments run smoothly!
How to Handle Floating Neuronal Cells Upon Receipt
1. Upon receiving the cells, do not open the bottle immediately. Place it in the incubator to stabilize for 4 hours.
2. Neuronal cells are sensitive to temperature and vibration, so it's normal for them to be floating upon receipt. If you are not ready to start your experiment, collect the floating cell clusters and return them to the original bottle for cultivation. Ensure the cap is loosened if the bottle is not breathable. For a T25 flask, leave 5-6 mL of culture medium.
Steps to Handle Floating Neuronal Cell Clusters
l Collect all cell suspension and centrifuge at 1200 rpm for 5 minutes. Retain the cell pellet.
l Add approximately 1 mL of 0.125% trypsin and gently resuspend the cells without pipetting up and down vigorously. Place the cells in a 4°C refrigerator to digest for 3-5 minutes.
l After the digestion period, add 5 mL of complete culture medium to stop the digestion. Centrifuge again at 1200 rpm for 5 minutes.
l Remove the supernatant and resuspend the pellet in 5 mL of complete culture medium (you may add 1% FBS to promote adhesion). Seed the cells into a new culture flask that has been coated appropriately.
l Keep the culture absolutely still for 24-48 hours. After 48 hours, observe the cells to ensure they do not cluster.
Important Culture Considerations
1. Neuronal cells cannot be passaged. Maintain the cells by changing the medium without passaging. Collect any floating cell clusters during medium changes.
2. Ensure that plates and wells are coated. Use diluted trypsin (0.125%) for digestion (dilute 0.25% trypsin with PBS) and digest in a 4°C refrigerator for 3-5 minutes.
3. Neuronal cells contract and detach easily when cold. Pre-warm all reagents to 37°C, and minimize observation time at room temperature.
4. Neuronal cells adhere loosely to surfaces. They are cultured and shipped in poly-L-lysine-coated containers, which may require longer or repeated digestion times for proper detachment.
5. Neuronal cells can remain viable for long periods but may gradually become contaminated with other cell types. For long-term experiments, it is recommended to begin within a week of receiving the cells.





