Cell Culture Academy| A Comprehensive Guide on How to Select and Use Serum
Source: PricellaPublished: 2025-03-14
In cell culture, serum is an essential reagent that directly influences cell growth and health. Therefore, selecting and using serum correctly is crucial. This edition of the Cell Culture Academy will provide a comprehensive overview of serum, delving into key factors to consider when choosing serum and addressing common challenges in cell culture, such as serum storage and thawing, the relationship between serum and microbial contamination, the causes of serum precipitation, and more. Solutions will also be offered to help streamline your experiments.
1. Serum Introduction to and Categorization
What is Serum?
Serum refers to the pale yellow, transparent liquid separated from blood plasma after the removal of fibrinogen and certain clotting factors following blood coagulation, or it refers to plasma that has already had fibrinogen removed (Figure 1). In cell culture, animal serum is widely used because it is rich in various nutrients that are essential for cell growth.
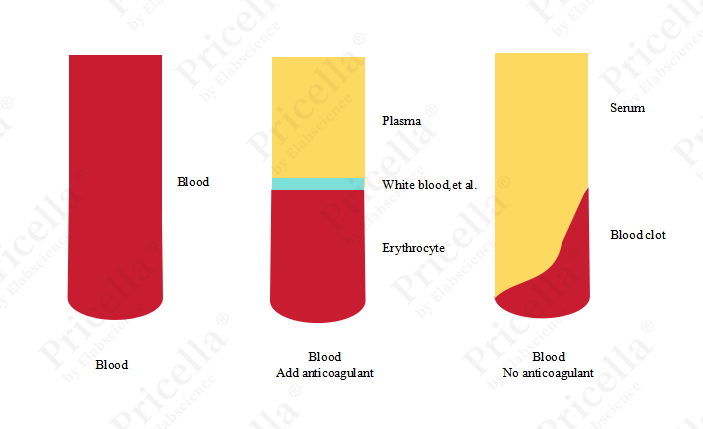
Figure 1. Diagram of Whole Blood, Plasma, and Serum
Categorization
Serum derived from various animal species can be categorized based on the species, including bovine serum, equine serum, chicken serum, rabbit serum, porcine serum, and others. Among these, fetal bovine serum (FBS) is particularly common and can be further classified based on the age of the cattle from which the blood is collected: fetal bovine serum, newborn bovine serum, calf serum, and adult bovine serum (Table 1). Fetal bovine serum, due to its unique advantages such as low antibody levels and high growth factor content, demonstrates distinct advantages in cell culture, although it is relatively expensive. To meet diverse experimental needs, various types of fetal bovine serum are available on the market, including natural low-IgG fetal bovine serum, stem cell-specific fetal bovine serum, and dialyzed fetal bovine serum, among others.
Table 1. Correlation Between the Types of Bovine Serum and Fetal Age
Number | Types | Age |
1 | Fetal Bovine Serum ( FBS) | Fetus (3-8 months of gestation in cows) |
2 | Newborn Bovine Serum (NBS/NCS) | NBS (Strict): within 24 hours of birth NCS (Broad): within 14 days of birth |
3 | Calf Serum (CS) | 16-22 weeks |
4 | Adult Bovine Serum | Adult Cow |
Serum is essential in cell culture. Since different experiments require different types of serum, it is recommended to choose the appropriate type based on the specific needs of the experiment. When necessary, a serum screening test can be performed for specific cells to identify the most suitable serum. However, in recent years, serum-free media have been gaining popularity. By adding growth factors and other components to mimic the functions of serum, serum-free media eliminate batch-to-batch variability and potential contamination risks, making them an inevitable trend in the future of cell culture.
2. The Role and Selection of Serum
Serum plays an essential role in cell culture. It is not only a critical nutrient for cell growth but also significantly influences the cells' state and function. Its main functions include:
providing the basic nutrients necessary for cell growth, such as amino acids, vitamins, inorganic salts, lipids, and nucleic acid derivatives;
supplying hormones and various growth factors that support cell growth and proliferation; providing binding proteins that carry important low-molecular-weight substances, ensuring their proper transport inside and outside the cell;
offering protection to cells by preventing damage from external environments, thereby creating a relatively stable growth environment for the cells.
When culturing cells, selecting the appropriate serum is crucial for optimal cell growth. Several factors must be considered when choosing serum:
(1) Cell Characteristics
Different cell types have distinct serum requirements. Most cell cultures are best supported by bovine serum, particularly fetal bovine serum (FBS). However, for certain cells, such as MCF 10A, NK-92, and undifferentiated PC-12, media containing equine serum are often recommended. Additionally, different cell types require varying serum concentrations. Typically, serum is used at a concentration of 10-20%, but for certain cell types, such as human bronchial epithelial cells and rat pancreatic ductal epithelial cells, media containing 2% FBS is commonly used.
(2) Experimental Requirements
When selecting serum, it is important to choose one that matches the specific experimental needs, particularly with respect to its nutrient composition and growth factor content, ensuring they are suitable for the cells being cultured. For some cell types, in addition to serum, specific growth factors or inhibitors may need to be added to regulate cell behavior, such as in the case of rat osteoclasts or mouse pulmonary microvascular endothelial cells. Even for the same cell line, such as BEAS-2B cells, serum requirements can vary depending on the experimental context. Therefore, whether to use serum-containing media should be determined by the specific experimental needs. In serum-containing media, BEAS-2B cells retain the ability to undergo squamous differentiation in response to serum (Figure 2). At low confluence, they exhibit an elongated spindle shape, and as confluence increases, the cells become more rounded. In contrast, in serum-free media, BEAS-2B cells show the characteristic polygonal shape of respiratory epithelial cells (Figure 3).
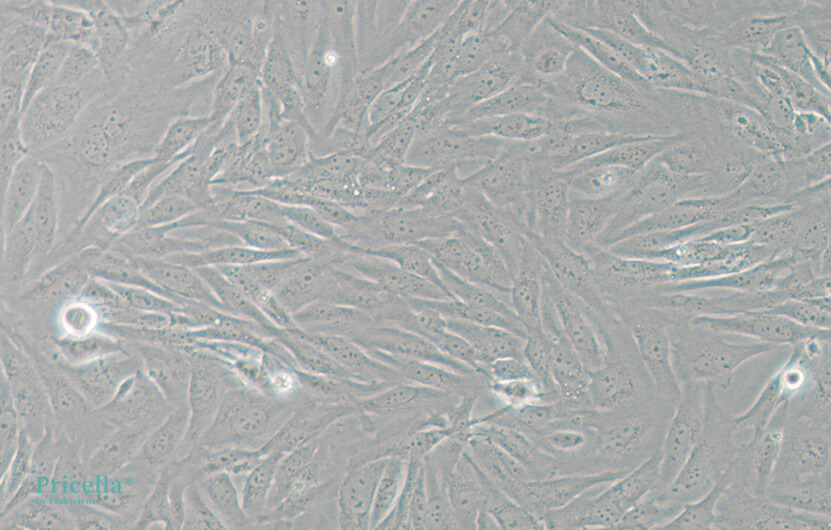
Figure 2. BEAS-2B in DMEM medium (containing 10% serum)
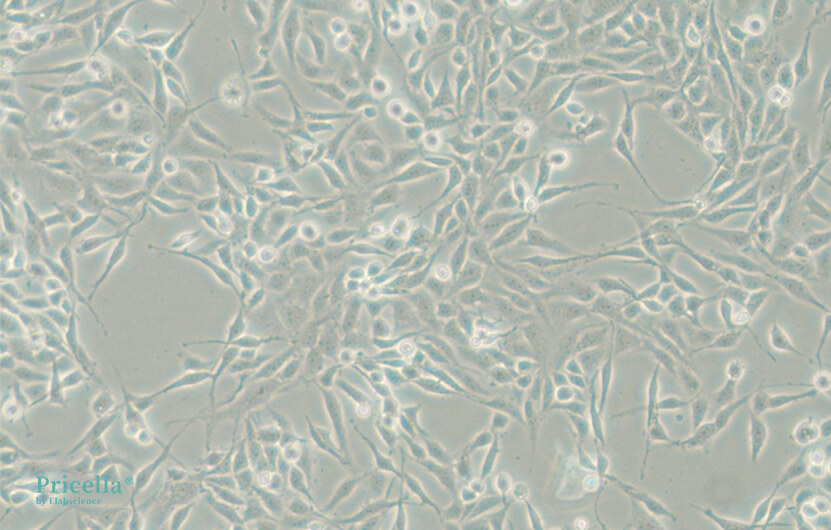
Figure 3. BEAS-2B in BEGM medium (serum-free)
The primary function of heat-inactivation is to reduce the activity of complement components in serum, or even to completely inactivate them. In certain cases, to avoid interference from complement proteins with cell growth and function, heat-inactivated serum is used for cell culture [1-3]. However, the heat-inactivation process may lead to the formation of precipitates in the serum, depleting its nutrients and negatively affecting cell growth. Therefore, heat-inactivation of serum is generally not recommended.
(1) Serum Quality
The source, production process, and packaging of serum can vary between manufacturers, so it is recommended to choose well-known brands with consistent quality. Due to the complex composition of animal serum, even within the same manufacturer, batch-to-batch variations can affect cell proliferation and morphology. For example, after switching to a different batch of serum, the morphology of L-929 cells showed noticeable differences (see Figure 4). Therefore, it is recommended to stick with the same serum brand and batch as much as possible and to store a sufficient supply of the same batch to reduce the impact of batch variation on cell culture. If changing serum is necessary, a gradual substitution method is recommended, transitioning step by step to minimize the impact on the cells. The specific procedure is as follows: First, mix 25% of the new serum with 75% of the original serum and culture for 1-2 passages. Then, mix 50% of the new serum with 50% of the original serum for culture. Next, mix 75% of the new serum with 25% of the original serum for culture. Finally, switch to using 100% new serum for culture.
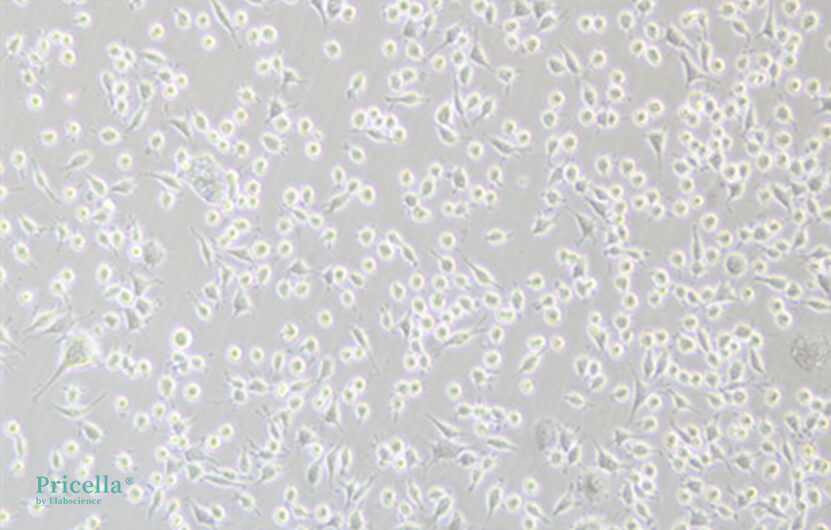
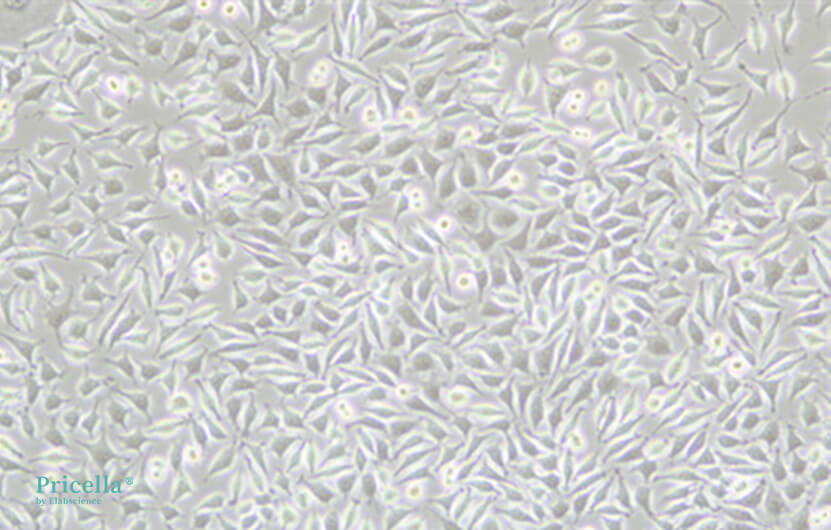
Figure 4. L-929 cells immediately after serum change (left), and culture one week later (right)
3. Common Issues in Serum Use
(1) Serum Storage
Serum typically needs to be stored frozen at -20℃ or -80℃ for long-term preservation. Improper storage can lead to a decline in serum quality, which may negatively impact experimental results. For situations where only small amounts are used at a time, it is recommended to aliquot the thawed serum and refreeze it to avoid degradation due to repeated freeze-thaw cycles. Thawed serum should be stored at 2-8℃ and used within one month, if possible.
(2) Serum Thawing
Serum should be thawed slowly at 2-8℃ to prevent degradation caused by rapid temperature fluctuations. During the thawing process, gently swirl the serum periodically to ensure uniform temperature distribution, prevent localized overheating, and minimize bubble formation, which could contribute to precipitate formation.
(3) Microbial Contamination
If serum is contaminated by bacteria, fungi, or mycoplasma, it will negatively affect cell growth. The serum’s condition can be checked by inspecting the integrity of the packaging, inoculating serum into culture medium to observe for colony formation, or using mycoplasma detection kits. Once contamination is confirmed, the serum should not be used, as its quality can no longer be guaranteed. Therefore, strict aseptic techniques should be followed to prevent microbial contamination during serum handling.
(4) Precipitates
Serum may form precipitates during the thawing process (Figure 5), which can appear as small black dots or fibrous deposits. Under the microscope, small black dots may exhibit Brownian motion. There are various theories regarding the cause of these precipitates: some believe it to be "nanobacterial" contamination. However, the more widely accepted explanation is that the precipitates are certain serum components, such as fibrin, calcium phosphate, or cholesterol, that have precipitated out. A small amount of precipitate generally does not affect the serum’s quality and can be slightly dissolved at 37℃ (but heating is not recommended). If the precipitates are more abundant, centrifugation (400 ×g, 3 min) can be used to remove them.



Figure 5. Serum is mixed during thawing (left), serum is not mixed during thawing, resulting in serum precipitation (middle), and small black dots appear when serum is not mixed during thawing (right)
Serum plays an essential role in cell culture and directly influences cell growth conditions. Therefore, when selecting serum, it is important to consider various factors, such as cell characteristics, the specific experimental requirements, and serum quality, to ensure the appropriate serum is chosen. Additionally, proper storage and thawing of serum, along with strict adherence to aseptic techniques, are crucial to prevent serum contamination.
References:
[1] Jin L, Duan Y, Li X, et al. High expression ITGA2 affects the expression of MET, PD-L1, CD4 and CD8 with the immune microenvironment in pancreatic cancer patients. Front Immunol. 2023;14:1209367. doi:10.3389/fimmu.2023.1209367.
[2] Zhang Y, Qiu S, Pang Y, et al. Enriched environment enhances angiogenesis in ischemic stroke through SDF-1/CXCR4/AKT/mTOR pathway. Cell Signal. 2024;124:111464. doi:10.1016/j.cellsig.2024.111464.
[3] Urzì O, Bergqvist M, Lässer C, et al. Heat inactivation of foetal bovine serum performed after EV-depletion influences the proteome of cell-derived extracellular vesicles . J Extracell Vesicles. 2024;13(1):e12408. doi:10.1002/jev2.12408.
Prev: L-929 Cell Culture Guide
Next: Master These Techniques to Distinguish Live and Dead Cells





